Introduction
PFAS, also known as “eternity chemicals”, can be present in wine. TFA (trifluoroacetic acid), a PFAS compound, has been detected in European wines, with concentrations increasing significantly since 2010. These chemicals are persistent, i.e. they hardly degrade in the environment and can enter the wine via various routes.

Trifluoroacetic acid (TFA) is a PFAS compound that is formed as a degradation product of other PFAS in the environment and can also occur as a contaminant in wine. The contamination of wine with TFA has increased in recent years, which indicates an increasing spread of PFAS in the environment.
The health effects of PFAS in wine are not yet fully understood, but the substances are considered potentially harmful and are suspected of causing health problems.
Intensive research is being carried out to identify the sources of PFAS contamination in wine and to develop strategies to minimize it. In summary, PFAS, particularly TFA, have been found in wine and concentrations have increased in recent years. It is important to understand the sources of these chemicals and take action to reduce their spread and minimize the potential health risks. This blog will emphasize a GC/MS method for the detection of TFA in wine that goes into the lower µg/l range.
Sample Preparation
Materials:
- Wine sample (red or white)
- Trifluoroacetic acid standard
- Derivatizing agent (diazomethane)
- Sodium chloride (NaCl)
- Sodium bicarbonate (NaHCO₃)
- Ethyl acetate or hexane
- Drying agent (e.g., anhydrous Na₂SO₄)
- Centrifuge tubes or separatory funnels
- GC vials
Procedure:
- Aliquot & acidify:
- Take 10 mL of wine.
- Acidify with HCl to pH ~1–2 to convert TFA to its protonated form.
- Salt out:
- Add a saturated NaCl solution to enhance extraction efficiency.
- Liquid-Liquid extraction:
- Extract with 2 × 5 mL ethyl acetate.
- Collect the organic phase.
- Drying:
- Pass the organic extract through anhydrous sodium sulfate.
- Derivatization:
- Mix with diazomethane
- React at 60°C for 30 min.
- Concentration:
- Evaporate solvent under nitrogen to ~0.5 mL.
- Reconstitute in hexane or ethyl acetate as a GC-compatible solvent.
- Transfer to GC vial:
- Filter if necessary and load into an autosampler vial.
GC-MS method for TFA analysis
GC Conditions:
- Column: DB-5MS, 30 m × 0.25 mm ID × 0.25 μm film
- Carrier gas: Helium, 1.0 mL/min
- Injection volume: 1 μL
- Injector temperature: 200 °C
- Injection mode: Splitless
- Oven program:
- 35°C (hold 2 min)
- Ramp at 10°C/min to 200°C
- Hold 5 min
MS Conditions:
- Ionization: Electron impact (EI)
- Scan mode: SIM or Full scan (m/z 50–300)
- Selected ions for TFA (derivatized):
- TFA Methylester derivative: monitor ions like m/z 69, 113, 195
- Use of internal standards (e.g., TFA-d1 or TFA-d3) is recommended for quantification.
Results
Fig.1: Shows a result for TFA Methylester in a wine sample on a single quadrupole in Full scan mode.
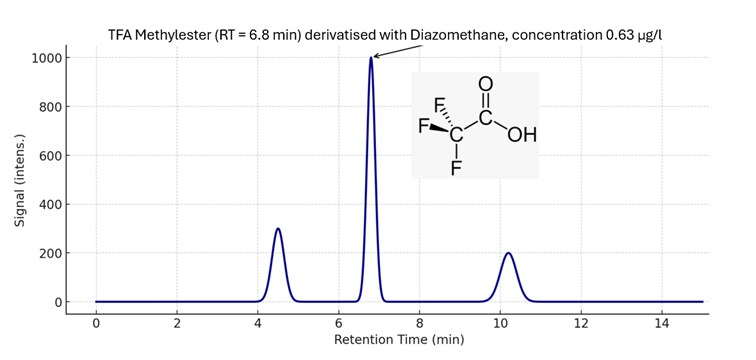
Fig. 1: FS of TFA on a single quadrupole
The chromatogram shows a strong signal for 0.63 µg/l TFA. If required, it would be possible to go down with the detection limit. Another way to increase sensitivity would be the use of Selected-Ion-Monitoring (SIM). However, sensitivity is not an issue on a GC/MS.
Modern wine samples (since 2010 or 2021-2024) contain conspicuous TFA values in the range of around 110-320 µg/L.
These are far above possible detection limits. As no TFA was detected before 1988, the TFA content at that time was below the (unspecified) detection limit, i.e. very probably below a few ng- to a few µg/L. Today, there is no limit from the EU for TFA in wine; however, research shows that even small concentrations (lower µg/l range) can be harmful to humans with certain diseases or a fetus.
Fig. 2 shows a calibration curve for TFA Methylester. In the real sample, 11.77 µg/l were found. Each calibration point equals 10 injections of the standard. The RSDs for the method were around 5 %.
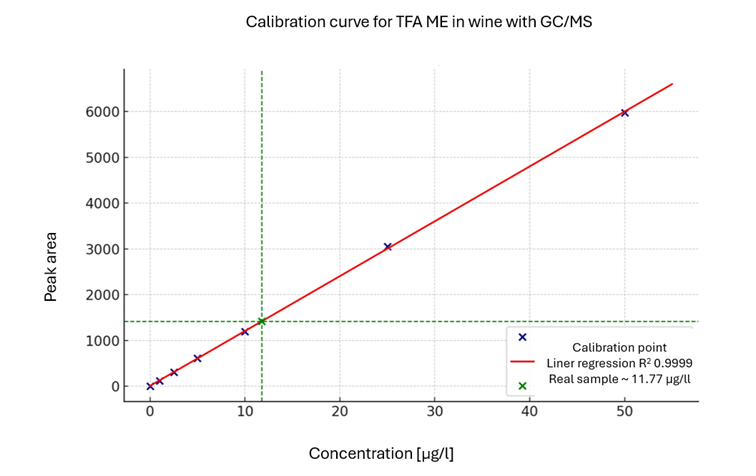
Fig. 2: Calibration curve
Conclusions
- High sensitivity
GC/MS can detect TFA at very low concentrations, which is crucial since TFA may be present in trace amounts in wine. - High selectivity and specificity
The mass spectrometer allows for specific identification of TFA based on its unique mass fragmentation pattern, reducing false positives. - Quantitative accuracy
With proper calibration, GC/MS provides highly accurate quantitative data, making it ideal for regulatory or quality control purposes. - Excellent resolution
GC can effectively separate TFA from other volatile or semi-volatile compounds in the complex wine matrix. - Matrix compatibility
Wine contains complex organic acids and alcohols; GC/MS can effectively separate and identify TFA despite the complex background. - Verification of contamination or origin
Detection of TFA could indicate contamination, industrial origin, or specific enological practices—information useful for quality assurance or authentication. - Automated & reproducible
GC/MS systems are highly automated and provide reproducible results, which is key for routine or high-throughput testing. - Dual functionality (Qualitative + Quantitative)
GC/MS offers both identification and quantification in a single analysis. - Regulatory acceptance
GC/MS is widely accepted in regulatory environments and scientific literature, making it suitable for official wine testing and export compliance.
Visit us on LinkedIn: #wine, #TFA #GCMS