Everyone is talking about PFAS, those persistent and toxic per- and polyfluoroalkyl substances! These compounds have been around since the 1940s, being present in products ranging from insulative materials in electronics to fire-fighting foams, industrial lubricants, consumer products, and fast-food packaging materials.

Estimates of the number of these compounds has varied, ranging from 9,000 to 15,000. Why such variability, you ask? Different regulatory agencies have different definitions of PFAS. For example, the Organisation of Economic Co-operation and Development (OECD) defines PFAS as any compound containing a fully fluorinated methyl or methylene group on an alkane functional group.1 In contrast, the U.S. Environmental Protection Agency (EPA) requires a PFAS compound to contain at least two adjacent, saturated carbons with one carbon fully fluorinated and the other at least partially fluorinated.2
There has been increasing interest in PFAS methods to determine their presence in various matrices. One of the most recently developed applications is for PFAS-related compounds using combustion IC, which is described in EPA Method 1621 Determination of adsorbable organic fluorine (AOF) in aqueous matrices by combustion ion chromatography (CIC). For this method, both single laboratory and multi-laboratory studies have been completed and it is now at the comment review phase.
The use of pyro-hydrolytic CIC has proven advantages. First, this is a screening method, not a speciation method. This means that you don’t need 15,000 reference standards, just a couple of fluoride stock standards and a couple of PFAS spiking standards. Second, CIC eliminates the sample matrix, resulting in an easy fluoride analysis by IC with suppressed conductivity detection.
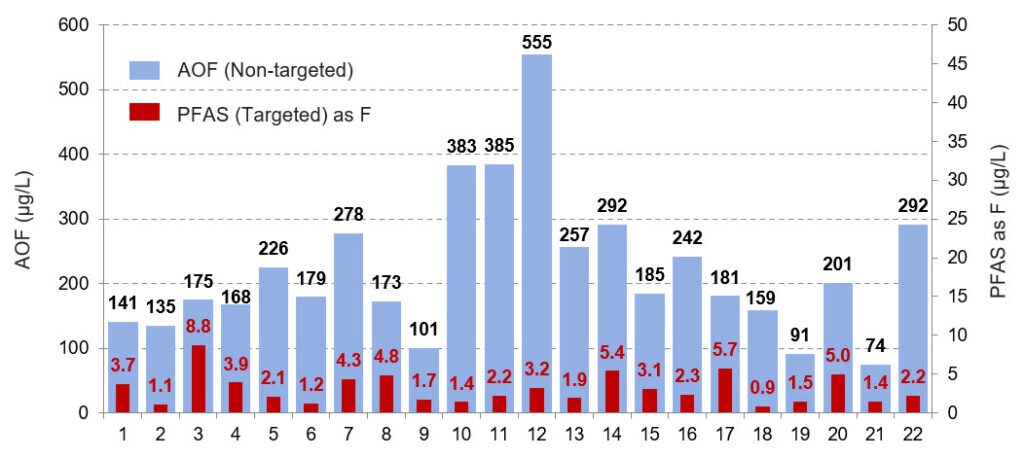
As noted above, there are thousands of potential PFAS compounds, and it is not practical to screen for each compound using a targeted approach. Screening by CIC gives you an aggregate value that can indicate if further, more targeted analysis is justified. This is readily illustrated in Figure 1, adapted from von Abercron et al.3 which shows the sum of fluorine from targeted analysis of wastewaters using LC-MS/MS overlaid on the values obtained from AOF determinations. The AOF values (blue) ranged from 30 to 175 times higher than results from the targeted analysis (red).
EPA Method 1621 has two sample preparation steps: adsorption and combustion. The wastewater sample is adsorbed onto granular activated carbon and rinsed to remove inorganic salts. The carbon is then pyrolyzed by combustion without oxygen at 950 ◦C, followed by hydrolysis in the presence of oxygen and water vapor at 1000 ◦C. For further reading, I’ve summarized my lab’s results in Thermo Scientific Application Note AN002748 along with a soon-to-be-published tips and tricks to succeed in implementing EPA Method 1621 in technical note TN003056. These documents are accessible via the AppsLab digital library.
EPA Method 1621 shows the advantages of IC and CIC ensuring the method is accurate and precise with process check standards. The method achieves sensitivity in wastewater samples of 2 ng/mL (ppb) fluoride. However, it’s important to note that drinking water analysis requires a different method and further discussion. I look forward to the further exciting developments with CIC that lie ahead!
References
1. OECD, Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance, OECD Series on Risk Management, No. 61, OECD Publishing, Paris, France. 2021.
2. European Fluorocarbons Technical Committee (EFCTC), U.S. EPA working definition for PFAS excludes TFA, EFCTC, Brussels, Belgium. November 2, 2021.
3. Adapted with permission: von Abercron, et al. Sci. Total Environ., 2019,673, 384-391. (https://www.sciencedirect.com/science/article/pii/S0048969719315876)
Visit our LinkedIn page #PFAS #Pesticides #CombustionIonChromatography