Part 1: A stellar way to overcome barriers for discovery verification
This is the first in a multi-part blog series highlighting the key pillars and impressive benefits of the Stellar mass spectrometer—the first-of-its-kind solution purpose-built for discovery verification. Stay tuned for more blog posts in the series.

If you were a handyman, you wouldn’t dream of using a wrench as a hammer, or a screwdriver as a chisel. You always need the right tool for the job. Same is true for clinical and translational researchers, who depend on having the right solution to maximize their productivity while advancing their research.
The challenge: A missing tool in the discovery-to-validation workflow
For decades, clinical and translational researchers have struggled with biomarker verification workflows due to a technology gap. There’s no current technology that simultaneously addresses the scale, sensitivity and throughput demands without some serious drawbacks. You know this well.
Triple quad technology performing multiple reaction monitoring (MRM) is challenged to keep up with limited capacity to handle massive target lists while quantifying at lower limits using faster LC gradients.
HRAM MS technology performing parallel reaction monitoring (PRM) are challenged to maintain high sensitivity with high acquisition speeds to function with high throughput at experimental scale.
The breakthrough solution: The first-of-its kind verification-class MS
Researchers struggling to fill the technology gap need not struggle any more. The first-to-market Thermo Scientific Stellar mass spectrometer expertly addresses the shortcomings of both ends of the existing verification-based mass spectrometer spectrum with its:
- Expanded scale – The Stellar MS expands biomarker candidate verification per unit time while covering a wider range of compound classes. For proteomics and metabolomics, the expanded scale enables researchers to include more stable isotope labeled standards (SIS) without having to drop endogenous targets, enabling absolute quantitation for better statistical analysis, plus method standardization.
- Higher sensitivity – It generates data incredibly fast, but still maintains excellent sensitivity to enable accurate measurements and better putative biomarker stratification. With its impressive quantitative performance, this mass spectrometer minimizes the risk of false negatives. The sensitivity of quantitation extends dynamic range in association with the expanded scale to provide both breadth and depth of sample analysis.
- Greater specificity – The Stellar mass spectrometer’s ability to acquire full scan MS2 and MS3 enables greater specificity by measuring all product ions associated with the precursor analyte, ensuring quantitative confidence in your targets of interest. It can detect smaller signal changes between donor groups (e.g., diseased and healthy), which ensures your measured expression levels are attributed to your target compound and not to background isomeric/isobaric interferences.
- Massive throughput – It provides the capacity to process all the samples in a study faster, which increases the laboratory capacity for more projects or expand the study size which increases statistical power for more confidence. Combined with the Thermo Scientific Vanquish Neo UHPLC system that delivers chromatographic performance from 0.1 to 100 µL/min, the Stellar MS has the acquisition speed to improve throughput 3-5X compared to methods using existing QQQ and HRAM instrumentation.
- Unrivaled quantitative productivity – Measuring a large number of targets is one thing, but how do researchers build these highly multiplexed methods? Traditionally, this process has been slow, painful and costly, requiring either direct infusion or many replicate injections followed by manual data evaluation. With the Stellar MS, researchers can go from building a method to completing an entire study faster while maintaining high data quality and confidence in results.
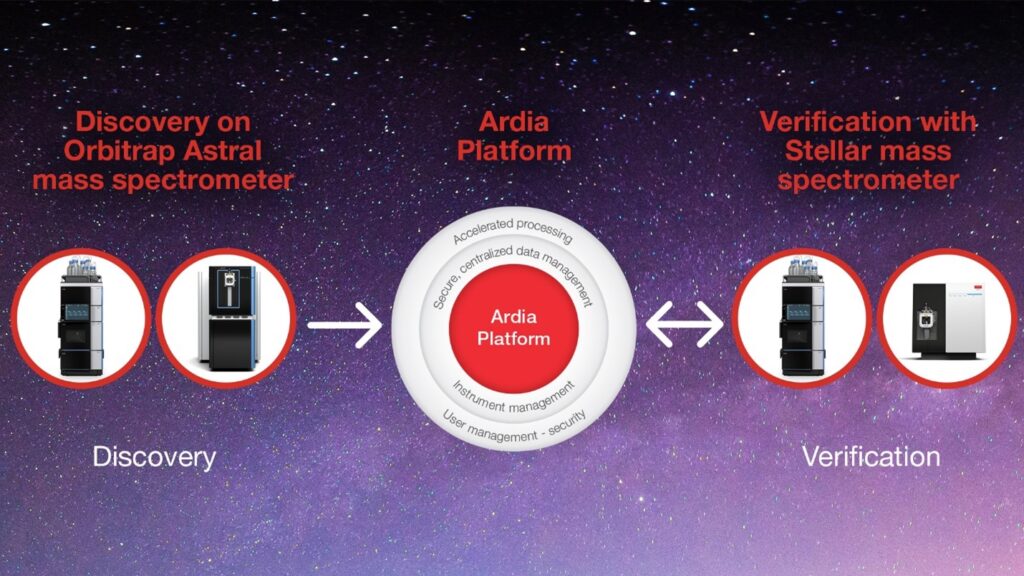
Bridging the gap between discovery and clinical validation
Not only does the Stellar MS represent a new class of instrument, it serves as the perfect “companion” product between the upstream HRAM instruments doing discovery and the triple-quad systems performing the downstream validation processes.
After discovery, the Stellar MS becomes invaluable for the verification phase—using quantitative accuracy to narrow the broad list of candidates with the highest potential down to the best set of markers to move into clinical validation. It can process a large list of potential candidates with the added benefits of higher sensitivity and quantitative accuracy.
Transitioning from verification to validation may deliver a narrowed set of biomarker candidates that have the greatest potential for specific and sensitive sample classification. Based on the Stellar mass spectrometer’s work, researchers now know the sample preparation steps, LC methods, retention times, detection range (LLOD/LLOQ) and method robustness that can be carried over to streamline validation.
In addition, researchers also have the option to directly transition the LC-MS/MS method to the Thermo Scientific TSQ Altis Plus triple quadrupole mass spectrometer if expanded laboratory capacity is needed. The ESI source, ion source settings, and Thermo Scientific Xcalibur software provide direct overlap to minimize additional method refinement. The TSQ Altis Plus MS also has the same robustness to ensure maximum uptime to complete studies with higher throughput.
No longer stuck in discovery: expanded capability across the entire workflow
Many translational researchers have avoided the move to targeted verification methods due to the complexity, as well as the lack of experience operating triple quadrupole systems to create, refine and implement these highly multiplexed methods. The introduction of the Stellar MS workflow streamlines the process while delivering quantitative performance.
Therefore, researchers can expand their laboratory capacity and advance their research work in house, without the need to add people, learn new skills or devote months to this work. Researchers can cover more of the workflow process themselves—all the way to the validation phase—enabling them to maintain better control, reduce costs and increase confidence in the results.
For more information, explore the Stellar Mass Spectrometer MS web page or reach out to your Thermo Fisher sales representative.
Intrigued to learn more about the Stellar mass spectrometer and how to streamline your verification process? Watch for more blogs in this series soon.
Visit our LinkedIn page: #StellarMassSpec #MassSpectrometry