Studying the causes and mechanisms of epileptic disorders
The human brain, for all its capability and potential, is ultimately controlled by a delicate balance of chemical signals. When these signals are disrupted, the consequences can range from minor symptoms to life-threatening disorders. Epilepsy describes a range of conditions where atypical brain activity causes seizures or other undesired behavioral symptoms. Researchers are constantly looking to deconvolute the causes and mechanisms of epileptic disorders in order to develop more effective, targeted treatments.
Neuron membrane proteins: a critical area of biomedical research
Speaking more specifically, neuronal signaling is regulated by the electrochemical activation of ion channels within the neurons’ cell membranes. Voltage-dependent potassium-ion channels, for instance, open in response to a particular membrane potential; the potassium ions pass through the channel in order to equilibrate the electrochemical gradient across the membrane, returning the cell to its resting state. This fundamental mechanism is what allows the neuronal cells of the central nervous system (CNS) to “reset” after communicating; it also makes CNS protein a critical area of biomedical research.
Using cryo-EM to better understand potassium channels and epileptic disorders
Voltage-dependent potassium-ion channels, particularly the Kv3 family that is encoded by the KCNC1 gene, have been implicated in a range of CNS diseases, including hereditary epilepsy disorders. Regulating their activation would require a clear understanding of their structures, as this reveals potential binding sites for therapeutic agents.
While crystallography has been the technique of choice for structural biologists, it is challenging to use on membrane protein complexes such as Kv3s, as they are resistant to crystallization. To overcome this barrier, researchers have turned to cryo-electron microscopy (cryo-EM) for structural analysis, as it can be used to analyze isolated proteins and complexes without the need for crystallization.
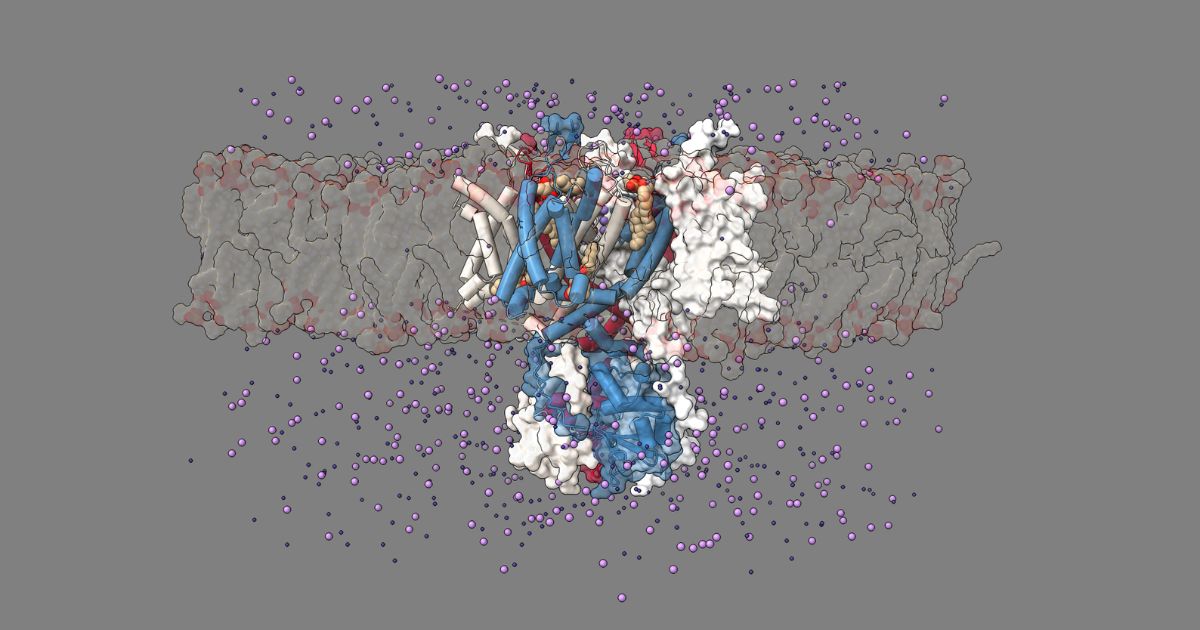
Kv3.1 in a membrane with potassium and chloride ions. Source: Katharina Dürr
Researchers determine high-resolution Kv3.1a potassium ion channel structure
In a recent Nature Communications publication, researchers at Oxford University led an effort to understand the structure of the Kv3.1a channel; mutations to this complex have been associated with severe epileptic disorders, but the exact mechanistic reason for this is still unknown.
Using cryo-EM, they were able to determine the structure of Kv3.1a to ~3.2 Å resolution. With this level of detail, they identified several binding sites for potential agonistic and antagonistic therapeutic agents. This included a previously unknown “control element,” a domain within the complex that has a significant impact on Kv3.1a channel gating.
These insights are a crucial first step towards structure-based drug design to alleviate the effects of several hereditary epileptic disorders. In a commentary piece that accompanied the research publication, CTO of Autifony Therapeutics Martin Gunthorpe shared this:
With respect to the translational potential of this work, it is encouraging that a number of companies are already developing Kv3 modulators and some of these have advanced into clinical development. In addition to the human genetically validated approaches identified, there are also additional opportunities in more prevalent diseases such as schizophrenia, Fragile X syndrome, and Alzheimer’s disease, supported by a growing understanding of the role of Kv3 channels in controlling neuronal activity in relevant cell types and circuits in the brain.
Read Cryo-EM structure of the human Kv3.1 channel reveals gating control by the cytoplasmic T1 domain in Nature Communications >>
Treating KCNC1-encoded ion channel disorders
Beyond just providing cryo-EM instrumentation for this project, our Thermo Fisher Scientific colleagues Mazdak Radjainia, Pu Qian and Pablo Castro-Hartmann worked with former colleague and now associate principal scientist at AstraZeneca Kasim Sader to collect critical data on the Kv3.1a complex in support of this research.
Their data resulted in high-resolution reconstructions of the channel bound to zinc ions. We are proud to play such an active role in this work and of the impact this research will have on therapeutic development.
Now that researchers have clear structures for this ion channel, they can investigate how they are impacted by genetic mutations to KCNC1. With a thorough understanding of the structural basis for Kv3.1a dysfunction, highly targeted treatments for epileptic disorders can be developed whose efficacy can be monitored down to the molecular level.
Recent studies highlight direct causal links between Kv3.1, Kv3.2 and Kv3.3 channel dysfunction and disease and it seems likely that it is only a matter of time for Kv3.4. These findings, together with the work of Chi et al., provide increased confidence in the validation and tractability of novel therapeutic approaches for Kv3 channels, including the development of precision medicines. It would therefore seem that the timing is right for recent advances in our understanding of ion channel structure, function and pharmacology to translate into new therapeutic approaches for channelopathies and other diseases with high unmet need.
-Martin Gunthorpe
Alex Ilitchev, PhD, is a Lead Scientific Editor at Thermo Fisher Scientific.

Leave a Reply