Cryo-EM: cryo electron microscopy
Cryo-electron microscopy (cryo-EM) describes a collection of techniques used to analyze cryogenically frozen samples in specially designed transmission electron microscopes (cryo-TEMs). Recent advancements, including improved sample preparation tools, detectors, and analytical computer algorithms, have even enabled the determination of 3D cryo-EM structures at atomic resolution.

Single particle analysis cryo-EM workflow.
How does cryo-EM work?
When samples are flash frozen (vitrified), the aqueous solution does not form crystalline ice, instead becoming a vitreous solid. This reduces the damage that the freezing process causes to the sensitive biological sample, thereby taking a “snapshot” of the specimen in solution.
Broadly speaking, cryo-EM techniques then image these samples at a variety of angles at high resolution and then digitally recombine the 2D snapshots into a 3D reconstruction. Cryo-EM can determine structures of challenging proteins and other macromolecules while providing wholly unique insights into how these proteins function. Although once considered a specialty tool used only by a small group of experts, cryo-electron microscopes are now increasingly easier to use, more affordable, and accessible to the wider scientific community.
Benefits of cryo-EM
Cryo-electron microscopy fills a complimentary niche next to other popular structural analysis techniques such as X-ray diffraction and nuclear magnetic resonance. Compared to other techniques, cryo-EM doesn’t require samples to be crystallized, which has been a significant barrier in the analysis of large complexes and membrane protein. Typically, all that’s required for cryo-EM is that the samples can be suspended in an aqueous environment.
Additionally, other techniques that require extensive sample preparation always carry the risk of subtly disrupting the biologically accurate structures as they exist in vivo. Through vitrification, this risk is significantly lowered, thereby providing physiologically relevant observations.

Comparison of common methods for imaging and structural analysis used in the life sciences, including cryo-electron microscopy techniques.
Cryo-EM techniques
While cryo-EM is sometimes used synonymously with the most popular technique, single particle analysis, there are a suite of analytical methods that can be performed in a cryo-TEM. We will provide a brief introduction to them here; far more information is available on our website.

Histamine in the binding pocket of human GABA-A (resolved at 1.7 Å with the Krios Cryo-TEM).
Data courtesy A. Sente and R. Aricescu, MRC-LMB Cambridge.
Single particle analysis
In single particle analysis, purified proteins or protein complexes are first vitrified to preserve their native structures. TEM is then used to collect numerous 2D snapshots of the samples. As the proteins are oriented randomly within the ice, these images show the sample at various angles, and can be recombined into a high-resolution 3D reconstruction of the sample.

Cryo-electron tomography visualization of a Golgi apparatus from the green algae Chlamydomonas reinhardtil.
Cryo-tomography
Cryo-electron tomography (cryo-ET), most commonly applied to whole cells, provides 3D visualization of organelles and protein complexes at nanometer resolution in their physiological environments. This is done by opening windows into the cell with focused ion beam (FIB) milling of a vitrified cell. A series of 2D images are taken of this thinned cellular sample, which are then reconstructed into a 3D dataset. Such high-resolution 3D images of the cell interior can provide new insights into cellular function and sheds light on the arrangement and structure of native protein complexes.
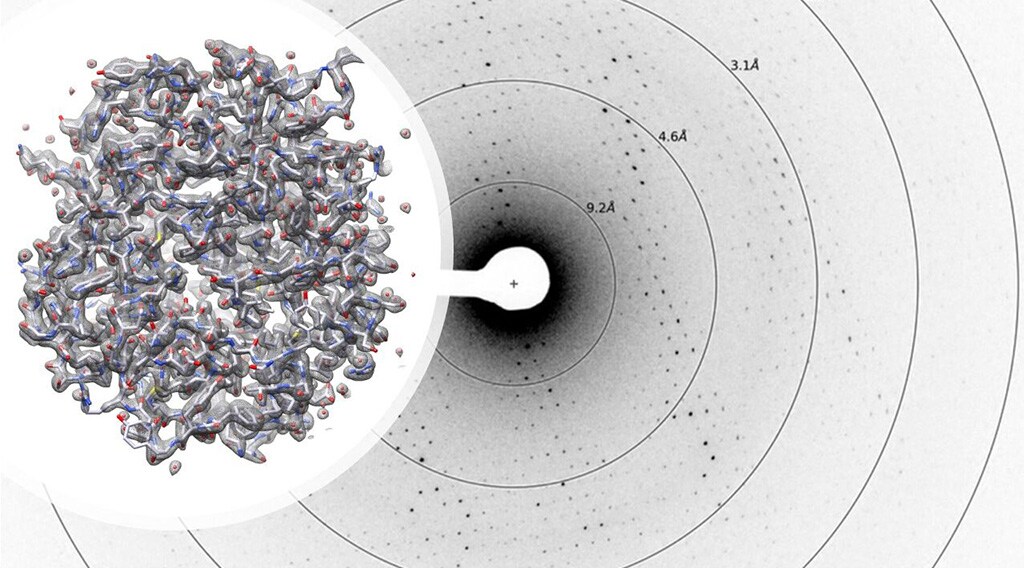
Protein structure determination with microcrystal electron diffraction.
Microcrystal electron diffraction
Microcrystal electron diffraction, or MicroED, is a diffraction technique similar to X-ray crystallography (XRD) that utilizes electrons rather than X-rays. Due to electron’s smaller wavelength and stronger interaction with the sample, atomic details can be extracted from individual nanocrystals (<200 nm in size), even in a heterogeneous mixture. This allows for the analysis of crystals that are too small for XRD and other crystallography methods. Data collection is completed in only a few minutes, and 3D structures can be determined at atomic resolution.
Conclusions
We hope this brief introduction has given you a glimpse into the power and possibilities of cryo-electron microscopy. With this revolutionary technology, structural biologists can analyze proteins in all their complex conformations, structures, and modified forms. They can even look at multiple protein conformations or heterogeneous complexes in a single sample.
If you’d like to know more about cryo-electron microscopy and the ways in which it is revolutionizing structural biology, pharmaceutical research, and more, please see some of our other educational posts on our Life in Atomic Resolution blog:
– Cryo-EM 101: Frequently Asked Questions
– Seeing with Electrons: The Anatomy of an Electron Microscope
– Open Access: Cellular Cryo-Electron Tomography Education
– Cryo-EM Sample Preparation: A Q&A with University of Utah’s Core Lab Director
For even more educational resources, visit our Life Sciences EM Learning Center >>
Alex Ilitchev, PhD, is a science writer for Thermo Fisher Scientific.

Leave a Reply