Edited on February 4, 2020.
Being HIV positive was, fundamentally, a death sentence as late as the mid-90s. This insidious virus, which was first characterized in 1981, has become a global epidemic, devastating its victims by destroying and overwhelming their immune systems. Without intervention this leads to AIDS, or acquired immune deficiency syndrome, which leaves the body incapable of fighting disease. Today, roughly 36.9 million people are infected with HIV, primarily in Africa. There is, however, hope; antiretroviral therapy (ART) is being used to reduce the viral load in those who are HIV positive, bolstering their immune systems and reducing their chance of spreading the disease to, effectively, zero. This is not, however, a cure, and the virus remains within the genetic code of the victim for the rest of their lives. In order to finally put an end to this disease, researchers now strive for an HIV vaccine that would prevent its spread altogether.
How HIV works
HIV, or human immunodeficiency virus, is a retrovirus, which means it introduces its genetic code into the genome of healthy cells to support its replication. In particular, HIV is a lentivirus, a subset of retroviruses that are characterized by extended incubation periods. Not only does this make the virus difficult to detect, hindering direct treatment, but it also means that the host person can spread the disease for many months, if not years, before they are aware they are carrying it. Once past its dormancy stage, HIV begins to attack CD4 immune cells (T cells), severely weakening the victim’s ability to combat any illness. AIDS is the final stage of HIV; it is at this point that individuals typically succumb to complications from other opportunistic diseases. HIV cannot be extracted from the host DNA after integration, which is why current treatments seek to lower the concentration of virus in the patient’s body, improving their T cell count and diminishing their ability to pass the illness to others. An HIV vaccine would, meanwhile, prevent the initial infection from taking hold at all, whether during virus fusion or integration.
Electron microscopy and an HIV vaccine
One of the key benefits of cryo-electron microscopy (cryo-EM) is that it can observe samples in their native environment without isolation and purification. This is done by vitrifying (flash freezing) the species prior to analysis, immobilizing the biological structures with minimal damage. This is particularly useful in the pursuit of an HIV vaccine, as the most promising antibodies target the intricate interaction between the host membrane and the virus.
These structures, called broadly neutralizing antibodies (bNAb), recognize and block a wide range of HIV types and prevent them from entering the cell. They do so by binding to various recognition sites on the HIV virus envelope. A recent study from the Department of Integrative Structural and Computational Biology at Scripps Research Institute in California showcased the utility of cryo-EM in monitoring the attachment of antibodies to their binding sites. With these “snapshots” of antibody behavior, researchers can clearly determine what vaccine candidates are working on a molecular level. Not only that, but electron microscopy (EM) could allow more rapid comparison of cross-species vaccine efficiency, accelerating the viability and success of human trials. A recent paper from Harvard Medical School was even able to capture, in extensive detail, the different conformations of the bound and unbound HIV envelope glycoprotein (see below). As this molecule is believed to initiate the fusion of the virus to the host cell, targeted obstruction of this mechanism is of critical interest.
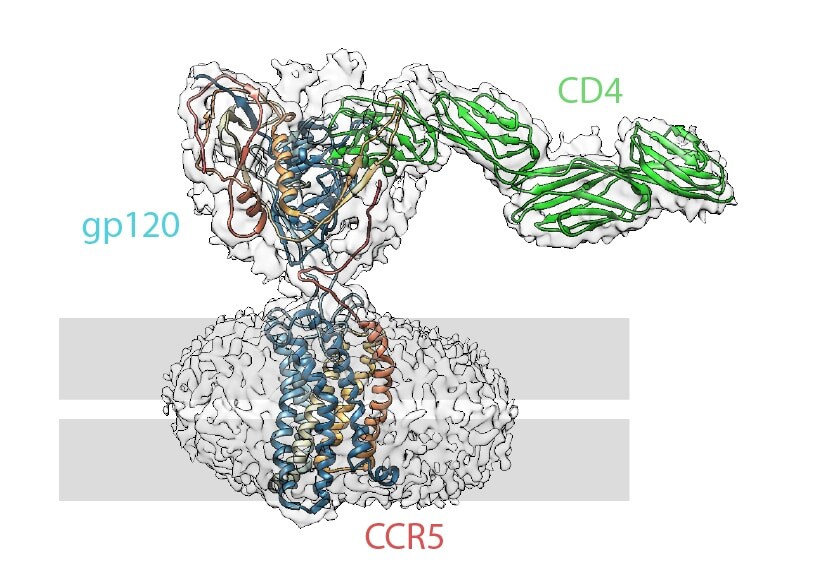
Structure of an HIV fusion protein complex with overlaid electron density map (clear outline). The location of the viral membrane is indicated in grey. Image generated based on the findings of Shaik et al. via the protein database (ID: 6MET), visualized by Chimera.
Another major avenue of research focuses on the incorporation of the virus into host DNA following virus attachment. This process is supported by the stable synaptic complex (SSC), which carries the virus DNA into the cell and then guides its integration. SSC tends to aggregate outside the cellular environment, meaning that the study of this structure through purification and crystallization is particularly difficult. Cryo-EM can circumvent these methods by imaging the SSC directly, once again leading targeted drug design.
Conclusions
HIV variants are highly structurally diverse, complicating the search for a broadly effective vaccine. The ways in which the virus and the host interact must be thoroughly scrutinized, in the native environment, in order to understand and iterate on potential drugs or antibodies. Cryo-EM provides an unparalleled look into these intricate mechanisms, driving research, development, and hope for an end to this devastating disease.
Subscribe now to receive new Accelerating Microscopy posts straight to your inbox. For more information about Thermo Scientific microscopy instruments, visit thermofisher.com/em.
Gabriella Kiss, PhD, is a Product Marketing Manager for Single Particle Workflow at Thermo Fisher Scientific.
To learn more about cryo-EM, fill out this form to speak with an expert.

Leave a Reply