If you are interested in studying neural cell markers, you will quickly realize that one of your biggest challenges may be in obtaining the “ideal” cell model. Primary neurons are generally hard to isolate and maintain in the lab and cell repositories carry few immortalized neuronal cell lines. A great way to overcome this problem is by generating neurons in the lab and here is where differentiation or cellular reprogramming can help.
Stem cells or progenitor cells serve as a bank to obtain specialized, functional cells of different types for a variety of applications as they possess the unique ability to differentiate into specialized cell types, when provided with specific environmental cues. Embryonic stem cells, fetal stem cells, adult stem cells or reprogrammed somatic cells (induced Pluripotent Stem Cells or iPSCs) are popular models for differentiation and the advantages they bring to research cannot be appreciated enough.
Let’s discuss why “DIY neurons” can be such fantastic tools for validating* antibodies. The Thermo Fisher Scientific R&D group uses embryonic stem cells and iPSCs to make several different types of neuron progenitor cells, which are further employed in antibody validation* and the data presented here will show you how.
iPSCs can be derived from a number of adult tissues, including skin and blood. These are essentially adult cells reprogrammed into stem cells, by over-expressing genes responsible for maintaining. They can be used to obtain a wide variety of terminally differentiated cells spanning the three derm-lineages. Since iPSCs are embryonic stem cell-like, they serve as an excellent source for generating specific cell models to study development in vitro, which in turn enables scientists to understand the expression and function of a wide array of proteins. Functional neurons – the cells in question – can be obtained from iPSCs using simple, scalable methods (1). They can also be used to generate specific types of neurons including dopaminergic neurons and glial cells (2, 3). Since in vitro differentiation of iPSC to specialized neurons mimics neuron development in vivo, scientists can use iPSCs to demonstrate changes in protein expression over the course of differentiation.
Additionally, iPSCs can be used to study expression levels of proteins in neurodegenerative conditions such as Parkinson’s or Alzheimer’s disease. Many labs report the derivation of patient-specific iPSC lines that can be used to generate neurons carrying disease-causing defects (4, 5, and 6). Such models can help scientists understand alterations at the genetic and/or protein level.
The key to making great antibodies is to validate them using relevant cell models and more importantly, by showcasing their specificity. Some considerations to keep in mind while choosing model lines to validate antibodies include the expression profile for the protein of interest and appropriate positive and negative controls. Cell lines and primary cells serve as excellent models to screen antibody libraries and validate them across species, but obtaining and maintaining these lines can prove to be laborious and time consuming. Also, scientists must factor in the possibility of alterations in the physical or genetic make-up of cells passaged over long periods of time in the laboratory. Often one may not find the “right fit” to validate antibodies – for example, as in the case of specialized neurons. This is primarily because most lines are derived from cancer cells, which in the case of neurons are usually from neuroblastomas that are a challenge to resect, or metastasized tumors from secondary tissue sources, which may be different from primary brain tumors.
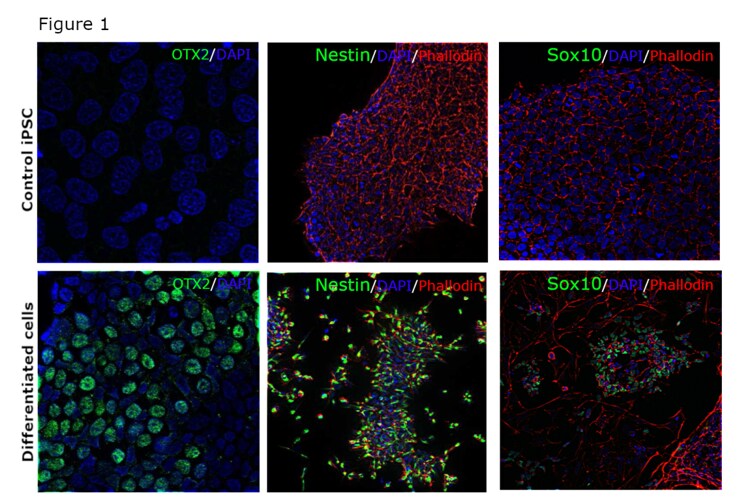
In order to circumvent these challenges and validate some of our neuron-specific antibodies, we differentiated iPSCs to neuro-epithelial cells, neural rosettes and Schwann cell progenitors. The methods we used have been previously described (1-11) and we were able to characterize these differentiated cell types using OTX2, Nestin and Sox10 antibodies, respectively, from the Thermo Fisher portfolio. As the data (figure 1) clearly demonstrates, we were not only able to confirm differentiation but also showcase, with confidence, the specificity of our antibodies as they only stained differentiated cells.
Figure 1: A, C, E – control iPSCs stained with antibodies against OTX2 (catalog number 701948), Nestin (catalog number MA1-110) and SOX10 (catalog number 703439); B – iPSCs differentiated to neuroepithelial cells and stained for OTX2 (green); D – iPSCs differentiated to neural stem cells and stained for Nestin (green); F – iPSCs differentiated to Schwann cell progenitors and stained for SOX10 (green).
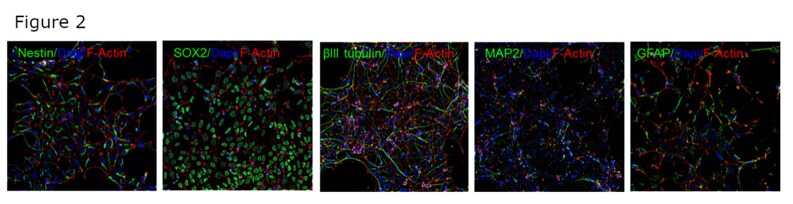
Embryonic stem cells (ESCs) can also be used to generate a number of different cell types including neurons. We used H9-derived ESCs to make Neural Stem Cells (NSCs) which were further differentiated into neurons and astrocytes. Antibodies against Nestin, SOX2, beta III tubulin, MAP2 and GFAP were used to verify differentiation of ESCs to NSCs, mature neurons and astrocytes (figure 2). The selective staining patterns of these antibodies suggest they are target-specific.
Figure 2: A, B – Human embryonic stem cells differentiated to neural stem cells, confirmed by staining with anti-Nestin antibody (catalog number 703439) and anti-SOX2 antibody (catalog number MA1-014); C, D – neural stem cells differentiated to neurons, confirmed by staining with anti-beta III tubulin (catalog number 32-2600) and anti-MAP2 (catalog number 131500); D – neural stem cells differentiated to astrocytes, confirmed by staining with anti-GFAP antibody (catalog number MA5-12023).
In addition to iPSCs, somatic cells can also be reprogrammed into neurons. Cell lines such as the neuroblastoma SH-SY5Y can be converted to mature neurons, eliminating the presence of epithelial cells and resulting in a homogenous neuronal culture (7). Neurite outgrowths, dopaminergic neurons and glial cells can also be rapidly obtained from lines such as Ntera-2 and PC-12 (8-11). Such models can be used to study signaling pathways and relative protein expression, and we routinely use them to validate antibodies specific to their intended applications.
It is estimated that poorly performing antibodies can waste up to 350 million dollars a year (12) and a major reason for this is the unavailability of the right screening models during antibody development.
By using model systems described, antibody suppliers can provide reproducible evidence to support the specificity of antibodies across applications such as ELISA, Western Blot, Immunocytochemistry and ChIP, thereby instilling confidence in customers looking to purchase the best available antibodies.
*This blog was authored by: Jamuna KS, Sreethu Sankar, Priyanka Swamynathan
Learn how Thermo Fisher is testing and validating antibodies
Search for antibodies
References:
1. Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation. D’Aiuto L et.al., Organogenesis, 2015; 11(2):93.
2. Directed differentiation of dopamine neurons from human pluripotent stem cells. Ma L et.al., Methods in molecular biology,2011; doi: 10.1007/978-1-61779-201-4_30.
3. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Abud ME et.al., Neuroresource, 2017; doi: 10.1016/j.neuron.2017.03.042.
4. Induced Pluripotent Stem Cells: An Innovative Patient-Specific Neurodegenerative Disease Modeling. Bonaventura G et.al., Stem Cell and Transplantation Biology, 2017; 2(2): 112.
5. Neurodegenerative diseases in a dish: the promise of iPSC technology in disease modeling and therapeutic discovery. Xie YZ et.al., Neurological Sciences, 2015; doi:10.1007/s10072-014-1989-9.
6. Modeling Human Neurological and Neurodegenerative Diseases: From Induced Pluripotent Stem Cells to Neuronal Differentiation and Its Applications in Neurotrauma. Bahmad H et.al., Frontiers in Molecular Neuroscience, 2017; doi: 10.3389/fnmol.2017.00050.
7. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. Shipley MM et.al., J. Vis. Exp. (108), e53193, doi: 10.3791/53193 (2016).
8. Rapid Differentiation of Human Embryonal Carcinoma Stem Cells (NT2) into Neurons for Neurite Outgrowth Analysis, Tegenge MA et.al., Cellular and Molecular Neurobiology, 2011; doi: 10.1007/s10571-011-9659-4.
9. NTera2: A Model System to Study Dopaminergic Differentiation of Human Embryonic Stem Cells, Schwartz MC et.al., Stem Cells and Development, 2005; doi: 10.1089/scd.2005.14.517.
10. Differentiation of the human NT2 cells into neurons and glia. Langlois A et.al., Methods in Cell Science, 1997; doi: 10.1023/A:1009731707443.
11. Assessment of PC12 cell differentiation and neurite growth: a comparison of morphological and neurochemical measures. Das KP et.al., 2004; https://doi.org/10.1016/j.ntt.2004.02.006.
12. Reproducibility: Standardize antibodies used in research. Andrew Bradbury and Andreas Pluckthun, 2015, Nature 518, 27-29; doi:10.1038/518027a.
*The use or any variation of the word “validation” refers only to research use antibodies that were subject to functional testing to confirm that the antibody can be used with the research techniques indicated. It does not ensure that the product(s) was validated for clinical or diagnostic uses.


Leave a Reply