For laboratories operating in regulated industries, compliance with stringent guidelines like ISO 17025, GMP, and 21 CFR Part 11 is challenging. Proving data integrity is a cornerstone of compliance, demonstrating reliable results and consistent testing processes and execution. For many labs, digitalization of lab data is a means to facilitate compliance. For others, the digital landscape serves as a barrier to data integrity.
Disparate informatics systems are the source of much of the difficulty laboratories face in demonstrating data integrity. Looking at the informatics infrastructure of most labs, you’ll find separate systems for Laboratory Information Management Systems (LIMS), Chromatography Data Systems (CDS), Lab Execution System (LES), Electronic Lab Notebook (ELN) and Scientific Data Management System (SDMS). Couple these systems with manual processes, spreadsheets, and paper-based methods and the goal of compliance can feel unreachable.
Building a Connected Lab
A connected lab infrastructure can help overcome the barriers to data integrity and compliance. Let’s look at chromatography testing as an example. In a non-integrated environment, multiple manual steps must be taken to accomplish any given task. You’ll need to pull sample information, enter sample lists and analysis in the lab notebook, update the CDS log, manually transfer results to a spreadsheet to do further calculations, approve those calculations and re-enter them in a lab notebook or into the LIMS. Each step in that process introduces the opportunity for error.
In an integrated environment, the process is much more automated. The workflow capabilities in Thermo Scientific™ SampleManager™ LIMS and Thermo Scientific™ Chromeleon™ CDS software support the digitalization of lab processes. The link between SampleManager LIMS and Chromeleon CDS enables exchange of information at user decision points. You can control the point at which data automatically transfers from Chromeleon CDS to SampleManager LIMS, ranging from immediately after a sample has acquired, to the end of a full review and approval cycle in Chromeleon software. As your SOPs are updated, you can easily manage and adjust your digitalized workflows.
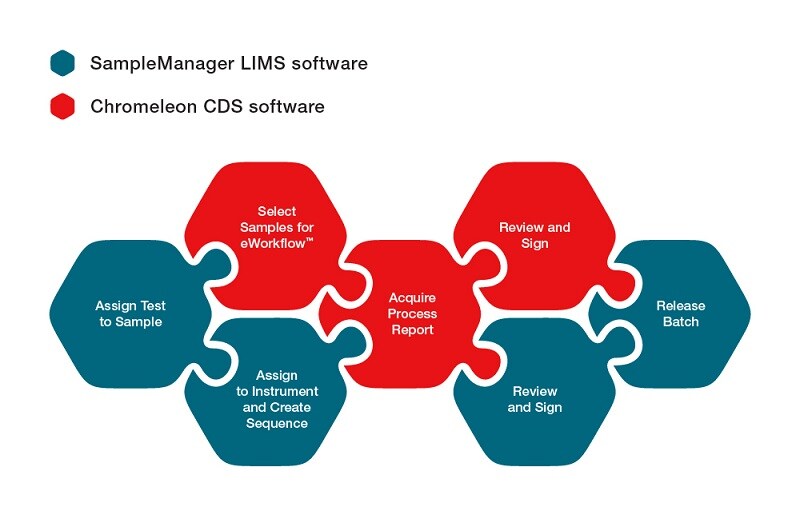
This connected infrastructure also enables the lab user to drive the chromatography testing process using their preferred solution. Experienced SampleManager LIMS users can drive the testing process without using Chromeleon software. Authorized users can assign samples to a range of instruments and sequences in a single process, and the link will automatically create all the sequences required to perform these analyses with the correct methods, sequence layouts and reports. Experienced users of Chromeleon CDS can also drive their mass spectrometry and chromatography analyses, pulling samples and automatically returning results, without having to run SampleManager LIMS software.
Linking LIMS and CDS
The link between SampleManager LIMS and Chromeleon CDS software connects LIMS and CDS workflows into a single, streamlined process. It enables access to all your chromatography and mass spectrometry data within the same environment, simplifying training of personnel and reducing operating costs. Your users can drive testing processes from their preferred system, increasing confidence and reducing risk of error. The link eliminates manual steps related to sample testing, results capture and analysis.
This is just one example of how a connected lab can help you prove data integrity and achieve compliance. Find out more at thermofisher.com/chromeleon or thermofisher.com/samplemanager. Or, download our SampleManager LIMS and Chromeleon CDS software link brochure.



Leave a Reply