
In just a few decades, chimeric antigen receptor T-cell therapy (CAR-T cell therapy) has undergone a meteoric rise into the spotlight as the pharmaceutical darling of 21st century oncology. It is the foundation of a rapidly expanding biotech field that has already supplied personalized cell therapy treatments to at least 1,000 patients in the US.
When cell therapy works, it really works. It’s common to hear stories of stunning cures in gravely ill patients with leukemia and other blood cancers who hadn’t experienced relief with any other treatment.
Still, among all the successes there are clear gaps. In building a new therapeutic industry from scratch, researchers and their manufacturing partners have learned a lot about what doesn’t yet work with CAR-T therapy.
A new class of therapy focused on CAR-engineered natural killer (NK) cells is now emerging in early trials to help fill these gaps – comparable enough to CAR-T to build off existing manufacturing approaches, but distinct in ways that really matter for wider access and better safety. And crucially, CAR-NK promises the same potential for significant therapeutic benefit, perhaps even beyond blood cancers and into the realm of solid tumors.
Could these natural killers be the key to safe, “off-the-shelf” allogeneic therapies that would crack open a major bottleneck for patient access to cell therapy?
We spoke with thought leaders from the product engineering side of cell therapy on the state of the field, what’s coming next, and why now is the right time to be excited about CAR-NK therapy.
What is CAR-NK therapy and how does it work?
Like T cells, NK cells are lymphocytes that originate in the bone marrow – NK accounts for 5-20% of these effector cells. But unlike T cells, which must first be primed to respond, NK cells are part of the innate immune system responsible for the body’s first line of defense.
As their namesake suggests, natural killer cells have an intrinsic cytotoxic capacity aimed squarely at the “non-self.” It’s a philosophical question for a single cell to grapple with but in the body’s language, the “self” boils down to a net activating signal mediated by MHC class I molecules acting as cell-surface ligands for NK receptors.
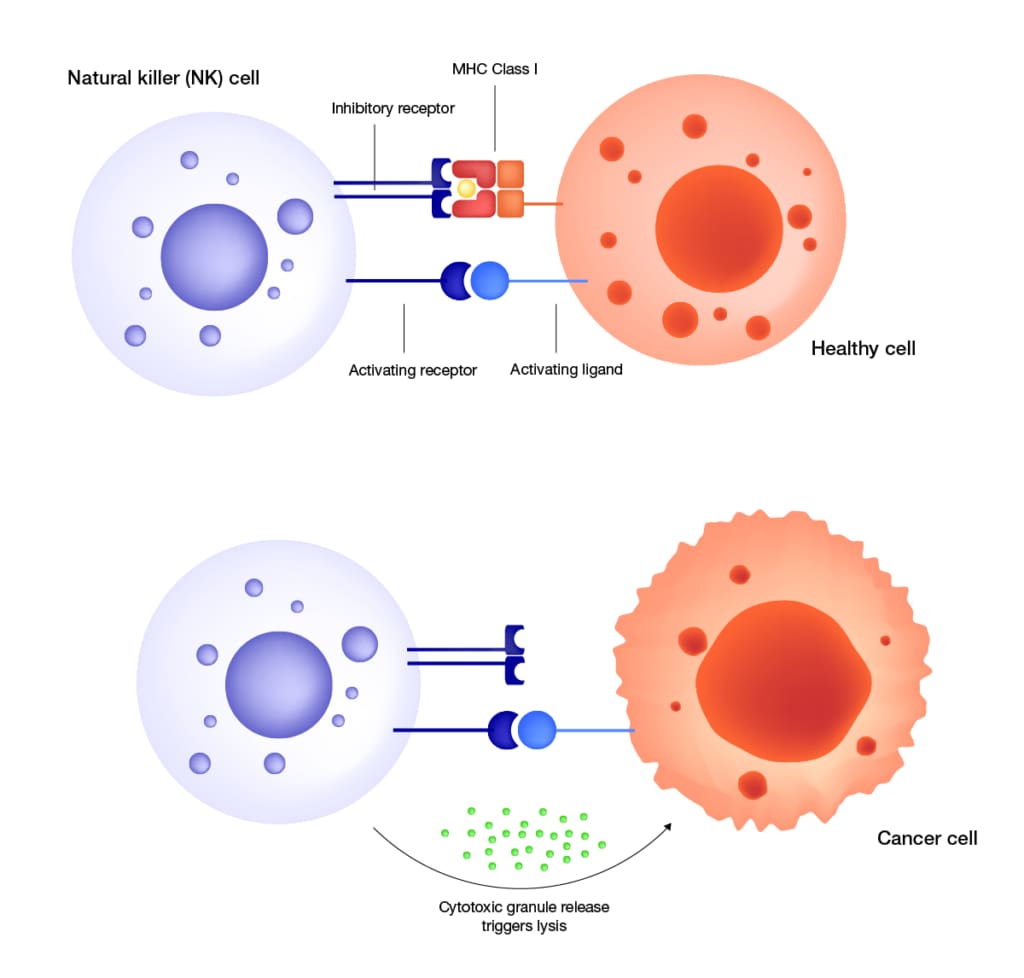
Natural killer (NK) activation mechanism. NK cells recognize target cells as healthy “self” through a net inhibitory signal mediated by MHC Class I molecules at the target surface. In cancer or infected cells that have lost inhibitory MHC Class I ligand signaling pathtways, NK signal balance shifts further towards activation via activating receptor induction. The activated NK cell releases cytotoxic granules to trigger target cell lysis. Like T-cells, NK cells can be enhanced with chimeric antigen receptor (CAR) expression for more targeted cell therapy applications.
“We’re talking about a balance of activation and inhibitory molecules at the surface. What’s different between NK cells and T cells is how they respond to MHC. Whereas MHC acts as an activation ligand for T cells to kill, it’s an inhibitory receptor for NK cells. With cancer or viral infection, the balance changes – the cancer cell is trying to avoid being killed by T cells, so it downregulates MHC expression. What the NK cell can then do is come along and say, ‘Wait a minute, every cell in the human body is supposed to express MHC. Something must be wrong.’ And it can kill the cell,” said Erica Heipertz, a cell biologist at Thermo Fisher Scientific who specializes in NK cell research.
“NK is like the sensor going around making sure everything is in balance. Once you get that out-of-balance surface molecule expression, NK will recognize it independent of antigen. It’s the check-and-balance system of the immune system,” said Heipertz.
You might close your eyes for a moment and think of this whole, complex dance unfolding at the molecular scale as not unlike your average office parking lot. Hired tow trucks scan the lot looking to remove any cars without an employee permit tag on the rearview – and so it is with NK cells patrolling to rid the body of cells lacking the proper MHC I credentials. T cells can also oust offenders, but someone would need to call ahead with the specifics – the license plate, make and model to look for (the antigen).
One big benefit of a natural killer, our in-house tow truck happy to intervene without instruction?
“They’re already killer cells – they’re already really functional. But if you can make them even more functional and targeted to a specific cancer, the therapy may not require as many doses or as many cells,” said Heipertz.
Another major upside to CAR-NK therapy lies in the fact that it has not triggered any severe side effects even when derived from donor cells, a key divergence from CAR-T therapy.1, 2
“What’s really cool about NK cell therapy is that so far, we haven’t seen severe side effects. In fact, many of the clinical trials for NK cells, from what I’ve heard, are being done as outpatient procedures,” said Heipertz.
Allogeneic therapy – or therapy produced from a donor cells rather than the patient’s own – has long been a challenge with CAR-T since it’s likely to trigger deadly graft-versus-host disease (GVHD) where native immune cells react against transplanted ones. Even autologous CAR-T therapy, the preferred method, can unleash fierce cytokine storms (clinically known as cytokine release syndrome) and serious neurologic side effects in a notable percentage of patients.
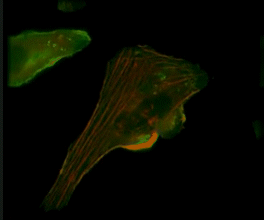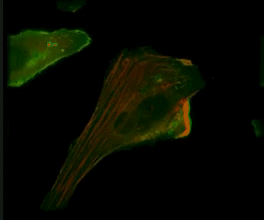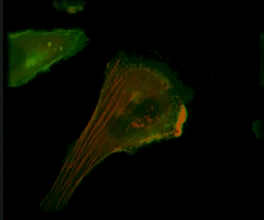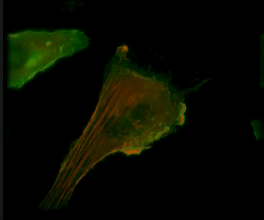
Live-cell imaging of an NK cell stained with Invitrogen CellLight Actin-RFP and GFP-centrin interacting with a target K562 cell stained with Invitrogen CellLight Actin-GFP.
NK therapy does not appear to carry the same side effects or risk of GVHD even in allogeneic form, and it requires none of the immune HLA mapping and matching that can take precious time and make it difficult to source a donor. Heipertz pointed out that preliminary evidence suggests that there may even be a therapeutic benefit to not matching donor and recipient HLA profiles.3,4
All of this adds up to another exciting option for treating blood cancers, and perhaps even solid tumors. There’s early hope that NK cells may migrate more easily than T cells to the tumor microenvironment.
“We collaborated with a group from the University of Maryland who had a mouse model with a skin tumor,” said Heipertz. “We infused NK cells into the blood, and the cells made it to the tumor site and controlled growth. It wasn’t completely gone, but NK cells did make it to the site. So I think there is some early indication that they could be effective for solid tumors.”5
Why CAR-NK over CAR-T genetic engineering?
The next question is, why now? We’ve known about NK cells’ role in preventing cancer since their discovery in the 1970s.
It was a dead end, perhaps, before the advent of personalized medicine and the revelation of adaptive immunity-based treatments engineered towards precise targets.
“Despite the centrality of the innate immune response, or perhaps because of its centrality, innate immunity has proven difficult to manipulate medically,” writes Siddhartha Mukherjee, cancer biology’s student-turned-unofficial biographer, in his book Song of the Cell.6
But there’s never been a more thrilling time to reconsider the simple savvy of the NK cell. The biology is there, the tools for manipulation are there, and the combined potential is incredible.
“The field really started with autologous cell therapy, and now we have ten years or so of research in that space,” said Xavier de Mollerat du Jeu, Senior Director of Research and Development in Cell and Gene Therapy at Thermo Fisher Scientific.
It has given NK cell therapy research and manufacturing a nice head start.
The cell therapy instrument manufacturing landscape, for example, has had time to improve from the early days of DIY ‘garage workshop’ style setups of individual instruments to factory-scale, fit-for-purpose, integrated manufacturing systems. Many key logistical aspects like cold storage and transportation are also improving in lockstep.
The R&D team at Thermo Fisher Scientific has been proactive in creating NK-specific protocols for the company’s high-throughput cell therapy instruments to adapt their use beyond T-cell applications. This includes the CTSTM XenonTM Electroporation System and CTSTM RoteaTM Counterflow Centrifugation System – both of which have been recognized as needle movers in their own right with major engineering innovation-focused awards like the Edison and the R&D100.

Even with these tools, scaling up cell therapy production to meet demand is no small task. If autologous therapy is the only option, it may be an insurmountable task. The average autologous CAR-T therapy course requires 3-8 weeks of manufacturing time, multiplied by some 60,000 patients in need annually.
From the patient’s perspective, that ‘vein-to-vein’ waiting period between cell collection and infusion can be more time than they have to spare.
“We quickly realized that this autologous CAR-T method works fantastically well but it’s extremely difficult to manufacture at scale. Allogeneic cell therapy is an incredible opportunity to streamline. It could also help serve as a ‘gap filler’ in allowing patients to recover and become ready for autologous therapy,” said de Mollerat du Jeu.
Thus the urgent need for mass production of reliable allogeneic cell therapy, an “off-the-shelf” alternative. NK fits that bill well.
“If we can figure out the off the shelf portion of it, if we can figure out a way to have a bank of these cells cryopreserved and available as a patient needs it? All you’d have to do is thaw and inject the therapy. That’s the big dream,” said Heipertz. “Patients won’t have to wait weeks and weeks for a biotech company to harvest and expand and engineer their own cells to give their treatment. Cell therapy should be something that one day is as readily available as a pill.”
NK cell expansion innovation
The Thermo Fisher Scientific cell and gene therapy team – Heipertz, de Mollerat du Jeu, and hundreds of their colleagues – are committed as partners to enabling scientists to manufacture next-generation allogeneic cell therapies at a wider scale than ever before.
For example, Heipertz was the lead scientist in developing the company’s CTSTM NK XpanderTM Medium specially developed to accelerate expansion of NK cells from peripheral blood, cord blood, or iPSC-derived sources in a feeder-free system.
“This media will help customers move away from feeders while still reaching that clinically relevant number of cells needed,” said Heipertz.
The use of iPSC-derived NK cells in particular is growing.
Learn more about NK cell therapy tools »
“We’re seeing a huge increase in the number of customers using iPSC-derived NK. Being able to have an efficient workflow and protocol that will allow generation of those cells I think is going to be really big. Whatever we can do to either speed up expansion time or make therapies more effective so that fewer cells are needed – that’s where the field is likely going,” said Heipertz.
“We’re enabling companies who want to manufacture an allogenic therapy by providing the tools they need,” she said. “We know that you’re going to need a very large number of NK cells for therapy. Unlike T cells, NK cells don’t really stick around for very long in the body. So it will take multiple doses and quite a large number of cells. Providing customers with a cell culture media with regulatory documentation, that can expand NK cells to a large number, I think really is one of the biggest puzzle pieces.”

Customers also inform the team’s innovation at every step.
“We worked with Dean Lee [Director of the Cellular Therapy and Cancer Immunology Program at Nationwide Children’s Hospital] when we launched NK Xpander. He’s doing clinical trials with NK cells and used the Xpander media to expand them. When we work with beta testers, we also get data to share with other customers.”
» Watch the Webinar with Dean Lee, MD, PhD: “Serum-Free Expansion of NK Cells with K562-Based Feeder Systems for Clinical Applications”
Building CAR-NK manufacturing infrastructure for the future
According to de Mollerat du Jeu, who calls thinking about these issues of cell therapy scaling “his life,” the job of manufacturing partners like Thermo Fisher Scientific is not to treat cancer. That role is for the academic researchers and clinicians of the world.
“They are the ones who decide what type of T cells they want to go after, come up with the antigen they want to target, and treat the patients. But to do all that, they need help. They need tools – new instruments, new reagents, new media that do not yet exist for a completely new type of therapy with living drugs. These kinds of tools may have been developed already but for other industries. For example, we do have 5,000 liter stainless steel bioreactors for things like antibody production. But for cell therapy manufacturing, you need one liter bioreactors,” said de Mollerat du Jeu.
It’s a strange paradox of shrinking down to scale up.
Traditionally, manufacturing systems are looming and large – even closed systems. Cell therapy is so much more individualized that it presents an entirely new scale of operations, and we need to miniaturize our tools to adapt.
And then we need to create a lot of them.
“The problem today is that many patients who are eligible for this therapy aren’t getting it because of different reasons like price, accessibility, and not having enough people and facilities in manufacturing. It’s not viable, in terms of square footage, and it’s only going to get worse,” said de Mollerat du Jeu. “There are so many potential applications for ‘living drugs’ like cell therapies, not just cancer. We have to resolve the manufacturing bottlenecks and simplify.”
“We need instruments that are integrated, automated, and have smaller footprints, so that you can put more into a facility and produce more therapy for the market. There are only six cell therapies approved on the market right now, but we’re projected to have as many as 25 annually in the next 5 years or so,“ he said.
That’s where next-generation, automated, closed-system instruments for scaled transfection, processing, sorting, and washing come in.

A researcher uses the CTS Xenon Electroporation system for non-viral transfection in cell therapy manufacturing.
It’s all part of a strategy is to build “fit-for-purpose” tools designed for this industry that cut out exposure to the environment and, with it, risk of exposing vulnerable patients to contamination.
“One of our main roles as a company is to help customer-collaborators transition their process,” said senior scientist Jason Isaacson. “Oftentimes, companies will have a lot of great scientists that know the biology and research but are not as familiar with how to translate it to closed systems and automation. We want to help them automate their systems as much as possible so they can feel more confident about an easier way forward with development.”
The workflows are complicated for even the most experienced scientists, and the Thermo Fisher cell therapy innovation team is happy to walk down that winding path side-by-side with customers. This includes finding ways to physically and digitally integrate individual instruments into workflow modules that are easily interchangeable to suit individual customer needs.
“We meet with our customers. We discuss data and help develop method improvements. We actually spend time in the lab with their methods and try to troubleshoot protocols, and we’re really available to hold their hand through the process of implementing instruments and reagents into their workflows,” said Isaacson.
Ensuring that workflows and instruments run smoothly, and that scientists feel empowered to use them to their full advantage, matters a great deal. There is no room for manufacturing error.
“You have one shot,” said de Mollerat du Jeu. “The patients give their cells and if you miss, if the manufacturing fails or you get contamination, that’s it. You’ve lost the cells and it could be very difficult to try again. We have to make sure the process is incredibly robust and sterile, and there’s no failure allowed. We need to make sure our tools are the best.”
Looking ahead towards CAR-NK therapy and beyond
It’s difficult to overstate how “astonishing” the results of cell therapy feel for the people who benefit, and for those who witness it as the miraculous revival of a gravely ill friend or family member.
It’s also difficult to overstate how devastating it can feel to researchers, patients, clinicians, and all involved to know that we could have the science arranged in its most crystalline form – the extraordinary solution to an extraordinary problem at last – but lack the means to deliver it. A crushing last-mile collapse.
“You have to understand, no one had ever seen these kinds of results before cell therapy. If you listen to discussions from the early days of CAR-T therapy, people were shocked that 13 out of 15 patients were cured in Phase I of trials. The thing that pains me is that we have these treatments for leukemia right now, and some people cannot have access. Kids can’t have access, because we’re having a hard time making it cheap enough and in enough supply. It’s a horrible problem,” said de Mollerat du Jeu.
“But at the same time, I really believe it’s a technology problem, it’s an engineering problem and through innovations we could truly make a difference. That’s what gets me excited. This is not about a drug that doesn’t work, it’s about making manufacturing viable. And that’s what’s driving us. We are incredibly lucky at Thermo Fisher Scientific to have basically the biggest toolbox in the world and the best people working on this technology. If we put our minds together, we really can fix this problem.”
We need all hands on deck to build better infrastructure and set the stage for a cresting wave of innovation – a revolutionary mix of accessible “off-the-shelf” therapies, new modalities of treatment (like NK cell therapy) mixed with improved applications of old, and immunotherapies that we may not have even uncovered yet.
Together, researchers and manufacturers are driving towards a brighter manufacturing future at incredible speeds.
“We know the end of the road, we know what these therapies can do, this is a problem that can be fixed. We know it’s there,” said de Mollerat du Jeu.
And one day, hopefully, cancer will be in the rearview and shrinking out of sight at last.
Learn more about cell therapy at thermofisher.com/celltherapy »
Want to read more stories like this? Subscribe to Connect to Science, your portal for life science news.
Further Reading on NK Cells and CAR-NK Therapy
- Immunology at Work: Natural Killer Cells Overview (Thermo Fisher Scientific)
- Cell Therapy Manufacturing: Protocol Handbooks, Virtual Lab, and more (Thermo Fisher Scientific)
- App note: Using CRISPR to Generate CAR-NK cells at scale (Thermo Fisher Scientific)
- “Current Perspectives on ‘Off-the-Shelf’ Allogeneic NK and CAR-NK Cell Therapies,” Heipertz et al. (Frontiers in Immunology, 2021)
- Connect to Science Profile: Building the CTS Xenon Electroporation System
- “Preclinical and clinical studies of CAR-NK-cell therapies for malignancies,” Li et. al (Frontiers in Immunology, 2022)
- Learn more about a day in the life of a leukemia research lab with Dr. Laura Eadie (Thermo Fisher Scientific)
##
References
- Liu E, Marin D, Banerjee P, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382(6):545-553. doi:10.1056/NEJMoa1910607
- Laskowski, T.J., Biederstädt, A. & Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer 22, 557–575 (2022). https://doi.org/10.1038/s41568-022-00491-0
- Liang S, Xu K, Niu L, et al. Comparison of autogeneic and allogeneic natural killer cells immunotherapy on the clinical outcome of recurrent breast cancer. Onco Targets Ther. 2017;10:4273-4281. Published 2017 Aug 28. doi:10.2147/OTT.S139986
- Wrona E, Borowiec M, Potemski P. CAR-NK Cells in the Treatment of Solid Tumors. Int J Mol Sci. 2021;22(11):5899. Published 2021 May 31. doi:10.3390/ijms22115899
- Adhikary, Gautam & Heipertz, Erica & Preradovic, Maja & Chen, Xi & Xu, Wen & Newland, John & Kaur, Navjot & Vemuri, Mohan & Eckert, Richard. (2023). Natural killer cells suppress human cutaneous squamous cell carcinoma cancer cell survival and tumor growth. Molecular carcinogenesis. 62. 10.1002/mc.23528.
- Mukherjee, Siddhartha. Essay. In Song of the Cell: An Exploration of Medicine and the New Human, 178–178. Scribner, 2023.
Intended use of the products mentioned in this article varies. For specific use statements, please refer to the product label.
© 2023 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
Leave a Reply