Search Thermo Fisher Scientific

| Katalognummer | Packungsgröße | Preis (EUR) | Verfügbarkeit | Menge | |
|---|---|---|---|---|---|
| A4738001 | 1 L | 646,00 | - |
Gibco CTS TrypLE Select is a recombinant enzyme capable of dissociating a wide range of adherent mammalian cells. CTS TrypLE Select Enzyme is derived from microbial fermentation and provides an animal origin free (AOF) alternative to porcine trypsin.
Gibco CTS products are designed for use in manufacturing of cell or gene products, supporting your transition from research to clinical applications.
Compared to other dissociation reagents, CTS TrypLE Select Enzyme is:
• Animal origin-free (AOF)—free of animal-derived components and formulated on dedicated animal origin-free equipment. CTS TrypLE Select Enzyme reduces risk of viral or prion contamination in your clinical manufacturing.
• Gentle on cells—CTS TrypLE Select Enzyme's exceptional purity increases specificity due to the action of a single enzyme. This reduces damage caused by cleavage from multiple enzymes in trypsin and other extracts.
• Room temperature-stable—Product remains stable at room temperature or 2–8°C, making storage and handling easy and convenient.
• Easy to use—CTS TrypLE Select substitutes directly into existing protocols, with no inactivation required avoiding the need for trypsin inhibitors, such as FBS.
The product is provided as a 1X stock solution, formulated in DPBS with 1 mM EDTA.
Closed systems compatible
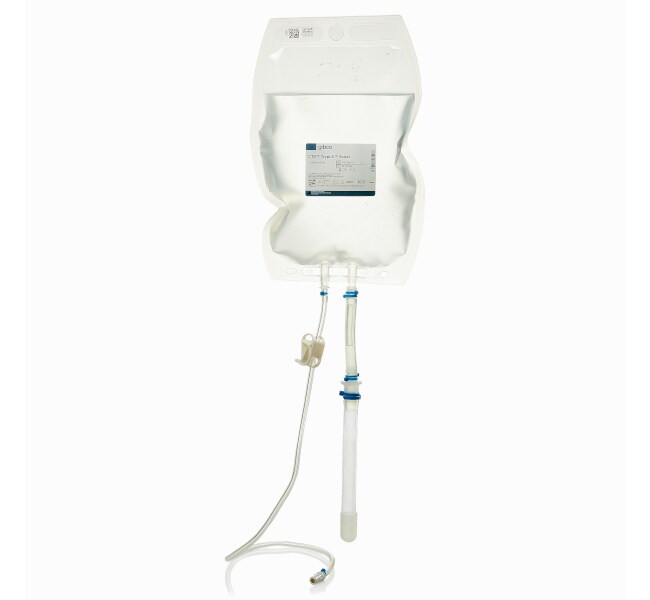
The 1-liter size of CTS TrypLE Select Enzyme is provided in bioprocess container format with the chamber constructed from Aegis 5-14 film. Sterile, weldable C-Flex tubing with female luer lock provides versatile connection options for integrating into a closed system manufacturing process.
CTS quality
The Gibco CTS product line enables you to reduce your burden in qualifying reagents during your transition from research applications to clinical applications.
Products are manufactured at a site that uses methods and controls that conform to cGMP for medical devices, 21 CFR Part 820. Our FDA-registered manufacturing sites are ISO 13485- and ISO 9001–certified. Gibco CTS products follow USP 'ancillary materials for cell, gene, and tissue-engineered products' within the responsibilities applicable to a supplier.
Gibco CTS products are of high quality and are supplied with harmonized documentation such as certificates of analysis, certificates of origin, and convenient access to our drug master file (DMF).