Authors: Haripriya Sridharan and Aparna Chandrasekaran
Research use only (RUO) antibodies are used in basic and applied research. Differing fundamentally in their end application from therapeutic or diagnostic antibodies, RUO antibodies are not meant for patient or clinical use; however, they are an essential component in a biotech or pharmaceutical scientist’s toolkit and are used in a myriad of ways during drug or diagnostic development. For example, they are extensively used as research tools to study biological processes and therapeutic targets, such as measuring protein levels after a drug treatment.
Advantages of recombinant monoclonal antibodies
One of the critical considerations for biotech or pharmaceutical research is antibody reproducibility. The traditional choice for monoclonal antibodies were hybridomas derived from the fusion of an antibody producing B cell with a myeloma cell line. However, hybridomas are plagued with challenges such as genetic drift, which can cause a drop in antibody titer over time.
Recombinant antibodies are a new generation of monoclonal antibodies developed in vitro by cloning immunogen-specific antibody genes into expression vectors. Since recombinant antibodies are defined by their sequence, they offer several advantages over traditional hybridomas, such as lot-to-lot reproducibility and the option to use an animal-free production system, in addition to specificity and sensitivity. Furthermore, recombinant antibody expression can be conducted at any scale in a high-throughput manufacturing environment along with a guaranteed long-standing supply of antibody, thus making them an excellent tool for testing multiple samples or for long-term studies.
Due to these advantages, many hybridomas are being converted to recombinant antibodies. During these conversions, the antibody encoding genes from the hybridoma cell lines are cloned into expression vectors. The recombinant antibody has the same antigen binding sequences as the parental hybridoma thereby, retaining the same antigen specificity as the hybridoma. For example, as shown in Figure 1, a rabbit hybridoma encoding an antibody recognizing somatostatin receptor 2 (SSTR2) protein was converted to a rabbit recombinant monoclonal antibody with specificity for SSTR2.
A)
B)
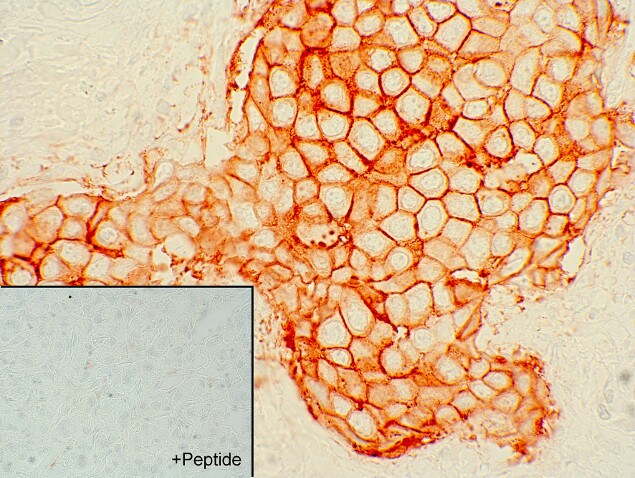
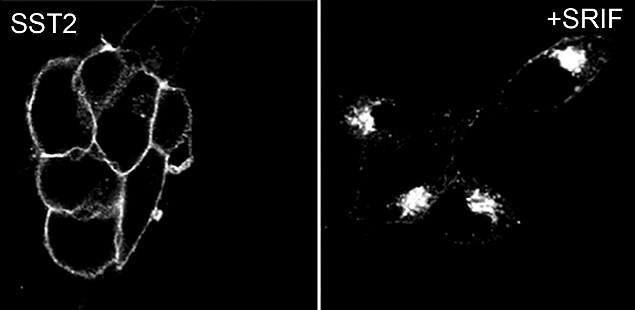
Figure 1: Recombinant antibody testing data for SSTR2 Recombinant Rabbit Monoclonal Antibody (Cat. No. 704011).
A) HEK-293 cells stably transfected with SSTR2, after 24 hours, cells were treated with 1 µM somatostatin-14 (SRIF) and then fixed and permeabilized. The specimens were incubated with SSTR2 Recombinant Rabbit Monoclonal Antibody (Cat. No. 704011, 1:1000 dilution). Cells were then incubated with Alexa 488-conjugated secondary antibody for 2 hours at room temperature, mounted and examined. Immunofluorescence analysis shows internalization of SST2 receptor from the plasma membrane to perinuclear cluster of the vesicles upon treatment with somatostatin-14 (SRIF – somatotropin release inhibiting factor). Altered expression of the protein upon cell treatment demonstrates antibody specificity.
B) Sections of human neuroendocrine tumor (NET) were dewaxed, microwaved in citric acid and incubated with SSTR2 Recombinant Rabbit Monoclonal Antibody (Cat. No. 704011, 1:1000 dilution). Sections were then sequentially treated with biotinylated anti-rabbit IgG and AB solution. Sections were then developed in DAB and lightly counterstained with hematoxylin.
Recombinant antibodies can be offered in a multitude of formats, including full-length antibodies from a single species or chimeric antibodies, as well as, antibody fragments such as single chain fragment variable (scFv) or antigen-binding fragment (Fab). Each of these formats offers unique benefits that are determined by their end usage and application. In the following sections, we highlight two such examples that were developed to serve distinct research needs.
A GPCR/G-protein complex stabilizing scFv recombinant monoclonal antibody
The scFv16 antibody fragment stabilizes active trimeric G protein complexes by recognizing the interface between G alpha and G beta/gamma subunits and is a valuable tool for CRYO-EM studies of GPCR protein complexes. This scFv is offered as a Recombinant Mouse Monoclonal Antibody (Cat. No. 703976) to serve customers focused on CRYO-EM applications of GPCR protein complexes (Figure 2). The product formulation was specifically developed to ensure compatibility for CRYO-EM applications.
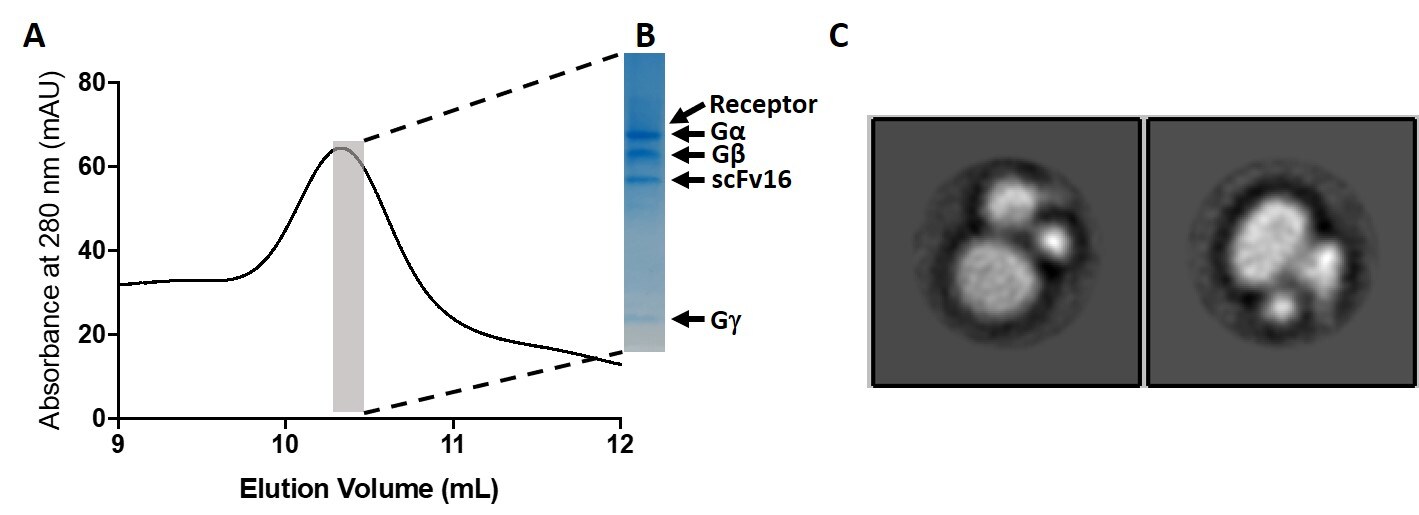
Figure 2: Recombinant antibody testing data for GPCR/G-protein complex-stabilizing scFv Recombinant Mouse Monoclonal Antibody (scFv16) (Cat. No. 703976).
A tagged GPCR was used to pull down a GPCR-heterotrimeric G protein complex that is stabilized by scFv16. A) Size exclusion chromatography (SEC) chromatogram of the purified complex. B) Highlighted sample from SEC was run on SDS-PAGE gel and Coomassie stained. All components of the active complex are present. C) Direct visualization of GPCR-G protein complex by negative stain TEM showing features characteristic for active GPCR complexes that are intact. Data courtesy Dr. David M. Thal, Monash Institute of Pharmaceutical Sciences, Monash University.
Recombinant antibodies for SARS-CoV-2 Spike protein
Sometimes researchers may have specific species requirements for an antibody to ensure compatibility with the rest of the assay or product development process. In these cases, the antigen-binding region (variable regions or the Fab fragment) of the antibody can be fused to the backbone of the required species. Having the same antigen-binding region gives the researcher complete confidence that the antigen-binding specificity is retained. Recombinant antibodies that recognize the spike protein of SARS-CoV-2 were developed as either fully human or human-rabbit chimeric antibodies to serve different research or product development requirements (Figure 3). For example, antibodies with a human constant region are useful as controls for developing kits to detect and characterize immune responses to SARS-CoV-2. At the same time, many laboratories routinely use rabbit antibodies to understand viral biology using immunoassays. Since a key focus area of SARS-CoV-2 research was to study the neutralization of the virus, some of these antibodies were tested and found to neutralize the ACE2-Spike protein interaction (Figure 4), making them useful controls for these experiments. The development of SARS-CoV-2 specific recombinant monoclonal antibodies, including the application and specificity testing, is discussed in further detail in a separate blog ‘Specific and neutralizing recombinant antibodies to SARS-CoV-2’.
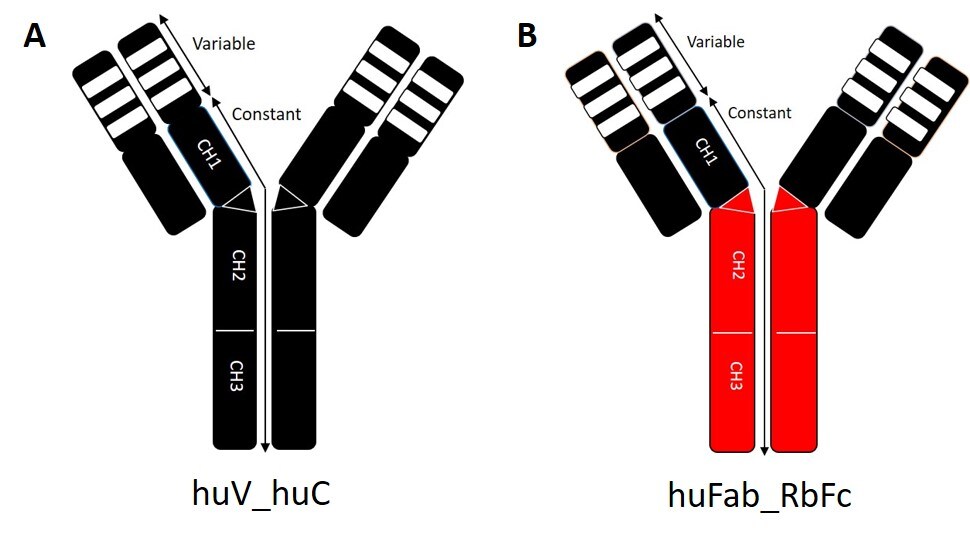
Figure 3: Schematic representation of the SARS-CoV-2 antibody backbones
A) Fully human backbone by grafting onto a human IgG1
B) Human Fab and rabbit Fc chimeric backbone by grafting onto a rabbit IgG.
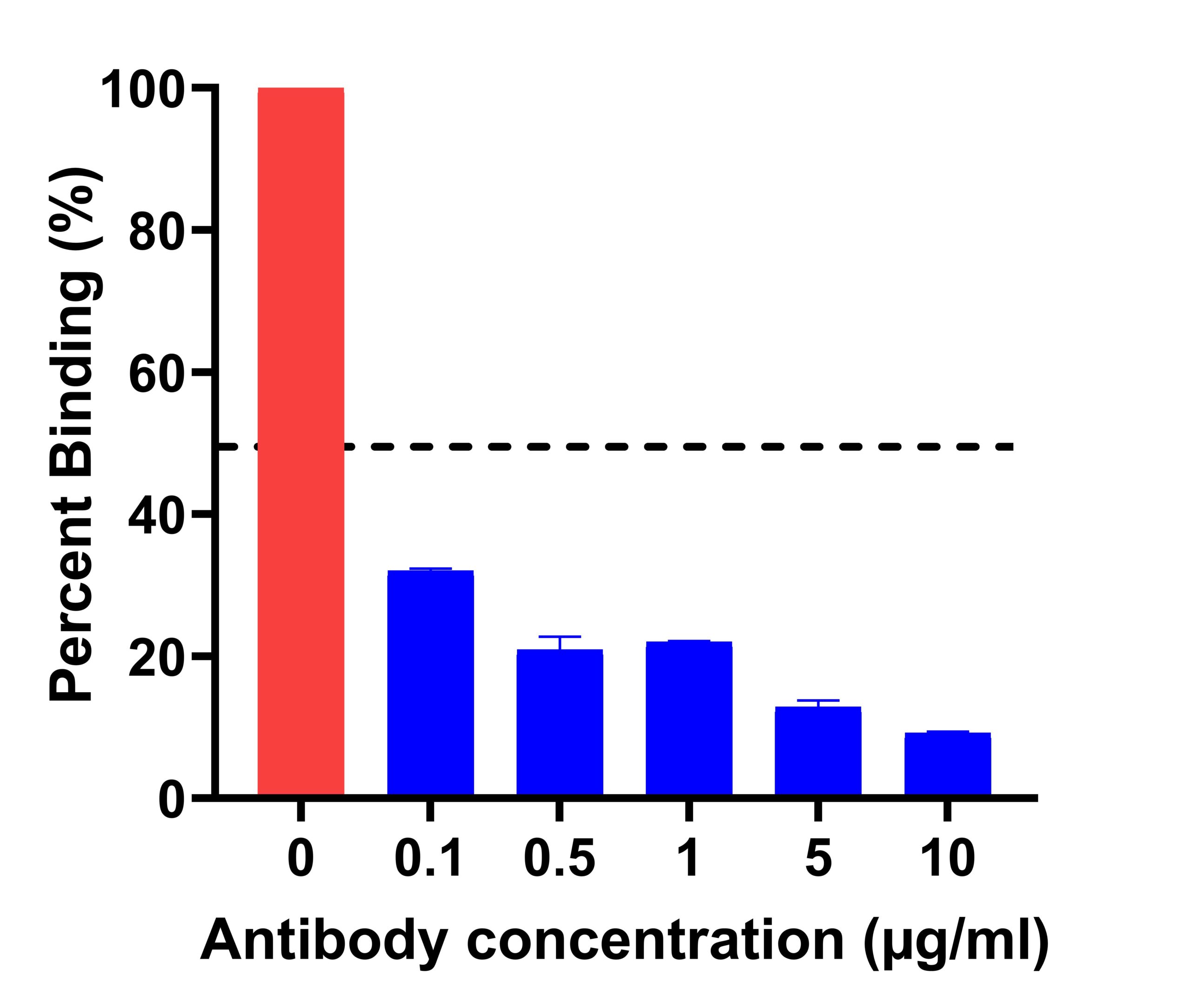
Figure 4: Spike protein RBD-ACE2 interaction blocking by SARS-CoV-2 Recombinant Monoclonal Antibody (Cat. No. 703973)
ELISA based SARS-CoV-2 inhibitor screening assay shows that the binding of SARS-CoV-2 Spike Protein RBD to human ACE2 was inhibited in the presence of the SARS-CoV-2 Recombinant Monoclonal Antibody (Cat. No. 703973). X-axis represents antibody concentrations and Y-axis represents percent binding signal of human ACE2 to SARS-CoV-2 Spike Protein RBD. The dotted line represents 50% inhibition.
RUO antibodies used in biotech and pharmaceutical research must often fit very specific criteria to maximize their utility as controls or reagents in various workflows. The examples described in this blog highlight how these requirements are being anticipated and met in order to provide optimal antibody performance and reproducibility to support the biotech and pharmaceutical research communities.
Additional Antibody Blogs:
Let’s get ‘specific’ about the TNFR pathway!
DIY Neurons for antibody validation
Translate to Invitrogen antibodies for your ribosomal protein research!
Drivers of the Chromosomal Passenger Complex
PRMTs: Role in epigenetic regulation
Using Blockers to Unlock Secretory Proteins
Specific and neutralizing recombinant antibodies to SARS-CoV-2
Staining Your Way into Cells: Exploring Cell and Organelle Markers


I didn’t know that recombinant monoclonal antibodies have advantages. I will be sure to try them. Maybe they could make a difference.