Many of our customers are embarking on a digital transformation journey or driving toward an Industry 4.0 strategy. We partner with these organizations to help them apply digital capabilities to realize transformational changes to their business. The term Pharma 4.0™ is a pharma industry focused interpretation of Industry 4.0 – focused around adopting innovative methods to deliver higher quality treatments to patients. In this blog, we’ll examine the intrinsic link between Pharma 4.0 and data integrity – and therefore the systems and technologies which organizations can use to drive these strategies.
Critical issues facing pharmaceutical manufacturers
To help understand the need for Pharma 4.0, let’s review the critical issues that pharma manufacturers have faced in recent years. Increasing compliance and validation requirements lead to more processes and documentation to provide evidence of the who, what, where and when of each action. There needs to be a way for analysts to be guided through complex processes, as well as an easy method to show that SOPs were correctly followed.
Connectivity is essential to bring together all the pieces of relevant data and information that could be helpful in successfully and safely bringing a drug product to market. The production and lab systems both hold critical data which are far more valuable as a complete piece – and providing all the relevant people in your organization with access to that data ensures that people are able to make fast, informed decisions.
There is an increased sense of urgency to deliver high quality drug products in as short a timeframe as possible. More tests are required but with the same number of analysts. This drives a real need to decrease the level of manual intervention that scientists are required to make throughout all processes, and to move toward modular manufacturing where continuous adjustments and improvements can be made based on in-line sensors and process monitoring.
Innovative technologies require new methods and increased regulatory checks – there is a need to ensure manufacturing organizations remain agile and able to adopt new capabilities to optimize manufacturing and deliver high quality products – and that’s where the data comes in.
The pharmaceutical ecosystem is also changing, especially considering what can be achieved with new capabilities. If we consider vaccine manufacturing as an example, the mRNA vaccines that we’re relying on to enable us to return to a new normal simply wouldn’t have been possible only a few short years ago, without the introduction of lipid nanoparticles as a drug delivery vehicle. Numerous other advancements including things like 3D printing have enabled more personalized treatments to be delivered to patients.
In combination with that, the increased use and reliance on digital technologies by various industries is clear. Our lives, our homes, our work, as well as science, continue to change rapidly as a result of technological advancements.
Pharma 4.0 refers to the specific application of digital capabilities to advance pharma manufacturing and deliver better products through innovative processes and techniques.
What is Pharma 4.0?
The Pharma 4.0 concept builds on Quality by Design and process analytical technology. Instead of waiting to check the product only at the end of the manufacturing process, Pharma 4.0 moves to real-time monitoring, using connected systems to enable a truly agile continuous manufacturing system where processes self-adjust based on the data being collected. Since data is at the heart of this system, it’s important to apply the latest data analysis techniques such as AI, ML and DL to enable simulations through which the system can learn and optimize product quality.
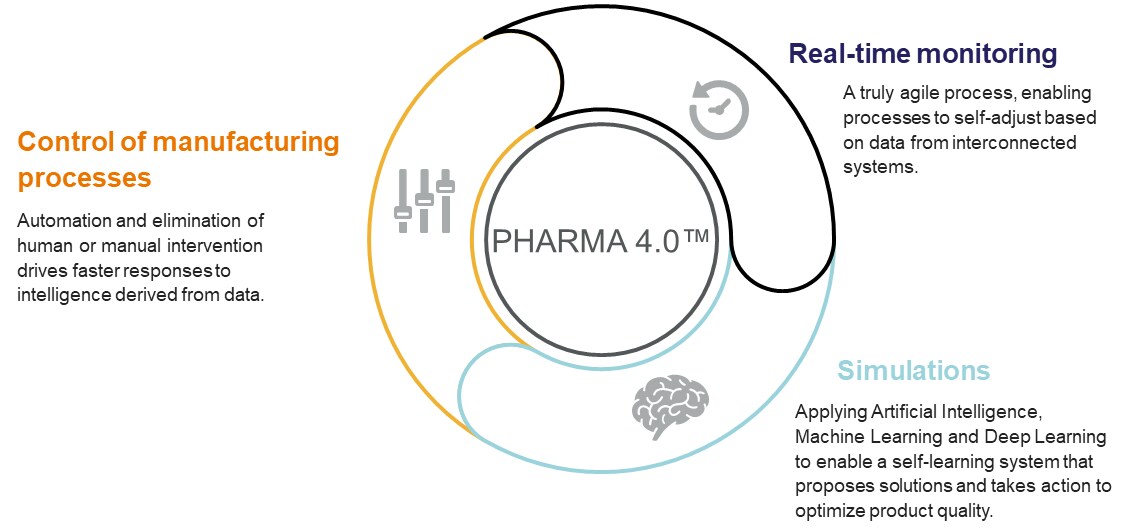
Pharma 4.0 looks to automate and eliminate any human or manual intervention by leveraging software to make decisions based on information collected from the connected systems and, where possible, automatically drive any actions such as changes to the manufacturing process.
This complete system creates a far more intelligent scenario, where pharmaceutical products are manufactured in a process which is constantly learning and proposing solutions to any issues that arise to enable optimum product quality.
Role of data integrity and digital maturity in Pharma 4.0
There are several things an organization needs to move to Pharma 4.0. This is the structure as defined by the International Society for Pharmaceutical Engineering (ISPE). The system is built on a certain level of digital maturity and has data integrity at its roots. 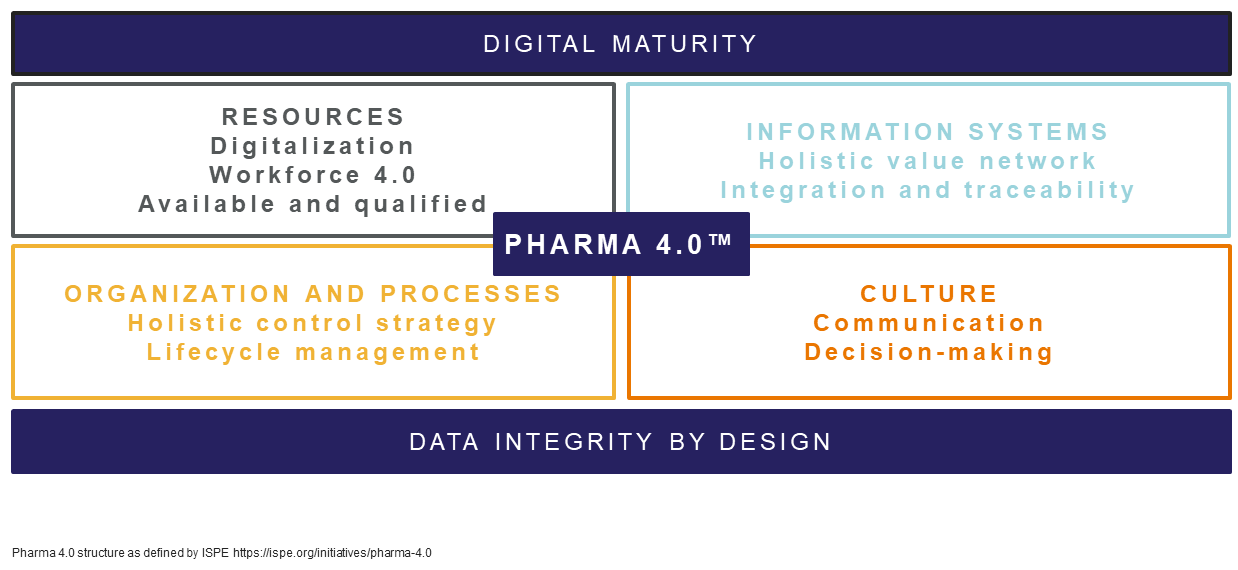
Looking at the resources required to achieve Pharma 4.0 and of course we are looking at a culture of digitalization to enable such an intelligent system. The workforce must be ready to accept this way of working, with an openness to change. The organization needs to have qualified experts ready to drive science forward.
The tools, devices, and IT systems need to be on an open platform to connect the necessary instruments and equipment, with common data standards and likely cloud-based to enable data sharing. The information systems are the basis of integration and traceability as well as automation, eliminating unnecessary manual or human intervention and reducing regulatory oversight.
A culture of strong communication, with a leadership keen to drive positive experiences through technology is also key.
Above all, data integrity is the common theme throughout Pharma 4.0, so organizations need to look at how this can be driven using the technologies and processes available.
Learning more on Pharma 4.0
If you want to learn more about Pharma 4.0 and data integrity, watch our Using Data Integrity to Drive a Pharma 4.0 Strategy in your Organization webinar on demand. Don’t forget to check back to the Connected Lab blog for future content on Pharma 4.0.

Leave a Reply