Sources of electrons
Nothing is more essential to the electron microscope than the electron beam, but all electron sources are not made alike. Understanding their basic function, as well as their respective benefits and drawbacks, is critical for choosing the ideal instrument for your experiments.
There are several commonly used sources, broadly divided into thermionic (tungsten filament, CeB6, LaB6) and field emission (tungsten tip – Schottky and cold FEGs):
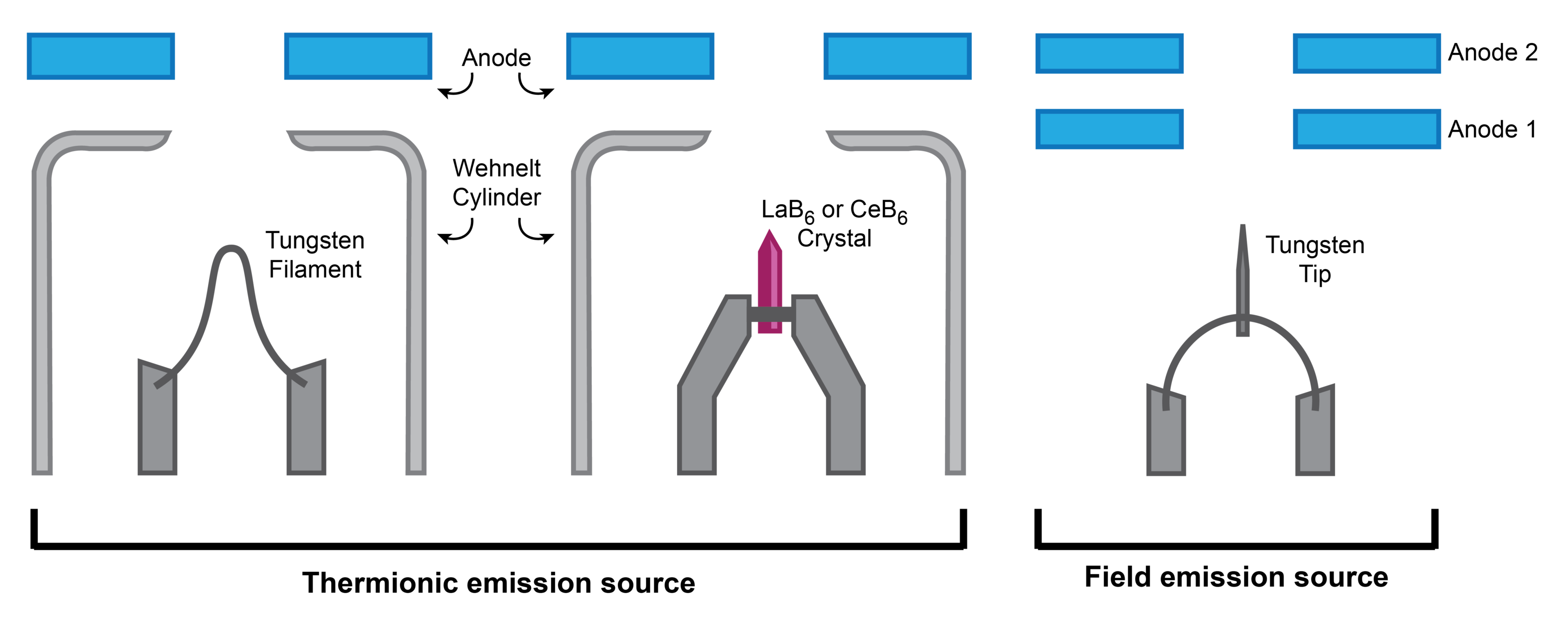
Illustration comparing the various electron emission sources. For thermionic sources the Wehnelt cylinder focuses the electrons as they flow toward the anode. In a field emission source, the first anode accelerates the electrons whereas the second anode focuses them.
Thermionic emission of electron
Thermionic sources rely on heat to generate electrons, similar to how light is produced by incandescent lightbulbs. As a current is applied to the filament (or crystal), it is progressively heated until its electrons have enough energy to escape the solid surface. However, unlike a lightbulb, the electrons must all flow in one direction to produce the beam, which is why an anode is placed nearby to attract the electrons and pass them on toward the column. (Note: here the electron source acts as the cathode, and it’s the voltage difference between the anode and cathode that accelerates the electrons forward.)
Tungsten filament: Tungsten filaments are comparatively cheap and easy to maintain; users can be taught to replace them, removing the need for ongoing external maintenance. Just like lightbulb filaments, however, they gradually lose mass to evaporation and eventually break, giving them the shortest lifetime of all the sources. Additionally, due to their high operating temperature, they have a lower brightness and a broader beam spread, resulting in generally reduced image quality (i.e. reduced image resolution).
LaB6 and CeB6: Lanthanum hexaboride and cerium hexaboride sources are composed of a single crystal of the respective molecule. Just like a tungsten filament, these crystals are heated by an applied current until there is enough energy to emit electrons. Compared to tungsten, lower temperatures are required to emit electrons, resulting in lower beam spread and higher brightness. They are also less volatile than tungsten and therefore have a significantly longer lifetime. However, they also need higher vacuum, thereby increasing the overall cost of the source.
Field emission electrons
Field emission sources (also called field emission guns or FEGs) use a strong electrostatic field to induce electron emission. This field is applied to the sharp tip of a tungsten wire, where quantum mechanical tunneling allows high-energy electrons to be released. The emission area is substantially smaller for an FEG (nanometers) than a thermionic source (micrometers), resulting in superior brightness and, in turn, enhanced image quality (i.e. higher spatial resolution and increased signal to noise). FEG sources also have the highest longevity, often lasting over a year without replacement. The primary downside of FEG sources is cost; the use of an electrostatic fields means that ultra-high vacuum is required, making it more expensive than most thermionic sources. Despite this, the increased resolution, brightness and lifetime of these sources makes them ideal for the widest range of applications.
Schottky FEG vs. cold FEG (CFEG): FEG sources can broadly be divided into Schottky or cold FEGs. As the names might imply, Schottky FEGs are thermally assisted, combining the benefits of thermionic and field emission sources. This is done by coating the tungsten tip in zirconium oxide, which facilitates the thermal emission of electrons when the source is heated. Note that although Schottky sources have a shorter lifetime than CFEGs and worse image quality under certain conditions (i.e. lower voltages, where the Schottky emitter has a larger energy spread), they generally have better stability.
To learn more about electron microscopy check out our other educational posts, such as “TEM vs. SEM: What’s the Difference?” and subscribe now to receive new Accelerating Microscopy posts straight to your inbox.
Alex Ilitchev, PhD, is a Science Writer at Thermo Fisher Scientific. Special thanks to Jake Jensen and Dustin Ellis for their help with this post.




I see Star Trek phasers in the near future. Its a beautiful time to be alive.