 A reader recently asked how to compute the output of “Rh” in grams if the sample quantity is 200 grams when using a precious metals analyzer that utilizes XRF technology.
A reader recently asked how to compute the output of “Rh” in grams if the sample quantity is 200 grams when using a precious metals analyzer that utilizes XRF technology.
XRF is a nondestructive testing technique that can analyze a metal sample in seconds with little to no need for sample preparation. Portable XRF precious metal analyzers deliver fast, accurate elemental analysis and karat value in seconds. In addition, handheld XRF analyzers are capable of distinguishing alloy grades that are nearly identical in composition to one another.
Rh is the symbol for Rhodium, number 45 on the Periodic table, which is a silvery white metal and a member of the platinum group metals (PGM). It is a highly active catalyst, and widely used in vehicle emission control systems. Rhodium can be alloyed with other metals to harden material and improve corrosion, and is also used to make glass thanks to its high melting point and temperature stability.
When the reader analyzed material with an XRF analyzer, he received the following result:
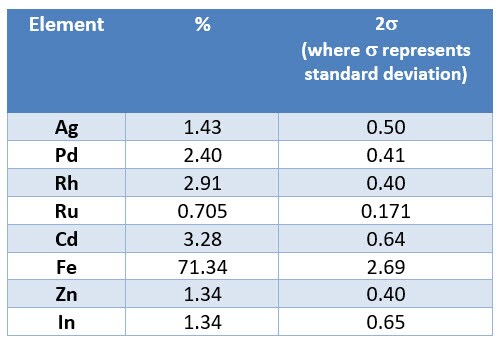
For the results screen provided by the reader, the elemental composition is reported in weight percent. To answer this user’s question:
The reported result on the screen for Rh = 2.91 wt.%.
Assuming homogenous material for all 200g of the sample, one would calculate Rh weight as 2.91 wt. % * 200g = 0.0291 * 200g = 5.82g.
To learn more about XRF technology, visit the XRF technology page on our website, or download the free ebook: Portable XRF Technology for the Non-Scientist






Leave a Reply