Search
Thermo Scientific Chemicals
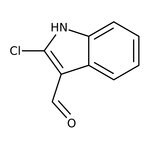
2-Chloroindole-3-carboxaldehyde, 97%
CAS: 5059-30-3 | C9H6ClNO | 179.603 g/mol
Catalog number H34381.03
also known as H34381-03
Price (JPY)Request A Quote
-
Quantity:
1 g
Chemical Identifiers
CAS5059-30-3
IUPAC Name2-chloro-1H-indole-3-carbaldehyde
Molecular FormulaC9H6ClNO
InChI KeyXYSSNBNFOBVMAU-UHFFFAOYSA-N
SMILESClC1=C(C=O)C2=CC=CC=C2N1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Cream to yellow to brown
FormPowder
Assay (HPLC)≥96.0%
Free acid (titration)≤1.5%
Identification (FTIR)Conforms
It is used as pharmaceutical intermediates. It is used in the synthesis of indole phytoalexin cyclobrassinon. And also in unprecedented chemical structure and biomimetic synthesis of erucalexin, a phytoalexin from the wild crucifer Erucastrum gallicum.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is used as pharmaceutical intermediates. It is used in the synthesis of indole phytoalexin cyclobrassinon. And also in unprecedented chemical structure and biomimetic synthesis of erucalexin, a phytoalexin from the wild crucifer Erucastrum gallicum.
Solubility
Slightly soluble in water.
Notes
Air sensitive. Store at room temperature.
It is used as pharmaceutical intermediates. It is used in the synthesis of indole phytoalexin cyclobrassinon. And also in unprecedented chemical structure and biomimetic synthesis of erucalexin, a phytoalexin from the wild crucifer Erucastrum gallicum.
Solubility
Slightly soluble in water.
Notes
Air sensitive. Store at room temperature.
RUO – Research Use Only
General References:
- H. D. Hollis Showalter.; Anthony D. Sercel.; Boguslawa M. Leja.; Craig D. Wolfangel.; Linda A. Ambroso.; William L. Elliott.; David W. Fry .; Alan J. Kraker.; Curtis T. Howard.; Gina H. Lu.; Charles W. Moore.; James M. Nelson.; Bill J. Roberts.; Patrick W. Vincent.; William A. Denny.; Andrew M. Thompson. Tyrosine Kinase Inhibitors. 6. Structure-Activity Relationships among N- and 3-Substituted 2,2‘-Diselenobis(1H-indoles) for Inhibition of Protein Tyrosine Kinases and Comparative in Vitro and in Vivo Studies against Selected Sulfur Congeners.J. Med. Chem. 1997, 40 (4),413-426.
- Justin R. Harrison.; Christopher J. Moody. The Horner-Wadsworth-Emmons reaction in the synthesis of macrocyclic peptides: the Trp-His-Gly-Arg derived macrocycle of moroidin. Tetrahedron Lett. 2003, 44 (28),5189-5191.