Search
Thermo Scientific Chemicals
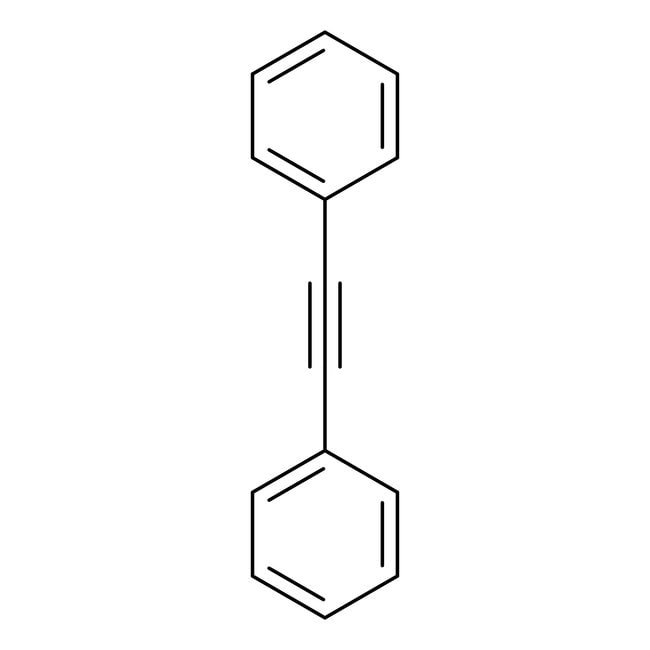
Diphenylacetylene, 99%
CAS: 501-65-5 | C14H10 | 178.234 g/mol
Catalog number A13979.14
also known as A13979-14
Price (JPY)Request A Quote
-
Quantity:
25 g
Chemical Identifiers
CAS501-65-5
IUPAC Name(2-phenylethynyl)benzene
Molecular FormulaC14H10
InChI KeyJRXXLCKWQFKACW-UHFFFAOYSA-N
SMILESC1=CC=C(C=C1)C#CC1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream
Assay (GC)≥98.5%
FormCrystals or powder or crystalline powder
Identification (FTIR)Conforms
Melting Point (clear melt)57.0-63.0?C
Diphenylacetylene is often used as a building block in organic and organometallic chemistry. It acts as dienophile and under goes Diels-Alder reaction with tetraphenylcyclopentadienone to prepare hexaphenylbenzene. It acts as a precursor in the preparation of 3-alkoxycyclopropene by reacting with benzal chloride in the presence of potassium t-butoxide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Diphenylacetylene is often used as a building block in organic and organometallic chemistry. It acts as dienophile and under goes Diels-Alder reaction with tetraphenylcyclopentadienone to prepare hexaphenylbenzene. It acts as a precursor in the preparation of 3-alkoxycyclopropene by reacting with benzal chloride in the presence of potassium t-butoxide.
Solubility
Miscible with ether and hot alcohol. Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
Diphenylacetylene is often used as a building block in organic and organometallic chemistry. It acts as dienophile and under goes Diels-Alder reaction with tetraphenylcyclopentadienone to prepare hexaphenylbenzene. It acts as a precursor in the preparation of 3-alkoxycyclopropene by reacting with benzal chloride in the presence of potassium t-butoxide.
Solubility
Miscible with ether and hot alcohol. Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- For an example of use as a Diels-Alder dienophile, see: Org. Synth. Coll., 5, 604 (1973).
- For transfer of methylene groups from dimsyl sodium, giving 2,3-diphenylbutadiene, see: Org. Synth. Coll., 6, 531 (1988).
- Can be oxidized to benzil using iodine-DMSO: Synthesis, 131 (1991).
- Shi, Y.; Li, G.; Zhao, B.; Chen, Y.; Chao, P.; Zhang, H.; Wang, X.; Wang, T. Synthesis and optical properties of cationic cyclopentadienyl iron complexes with diphenylacetylene chromophores. Inorg. Chim. Acta 2015, 427, 259-265.
- Le, K. V.; Aya, S.; Ogino, S.; Okano, K.; Araoka, F.; Takezoe, H. Large Twist Elastic Constant in Diphenylacetylene-Core-Based Liquid Crystals. Mol Cryst Liq Cryst 2015, 614 (1), 124-127.