Search
Thermo Scientific Chemicals
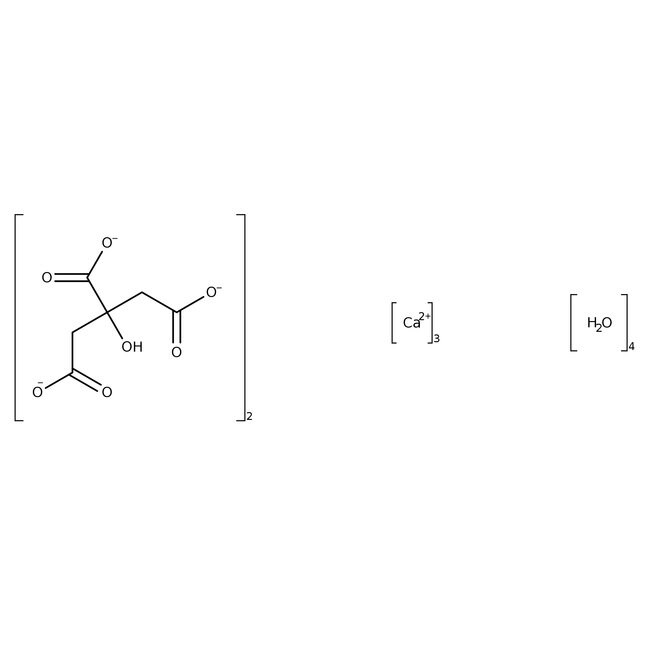
Calcium citrate tetrahydrate, 96%
CAS: 5785-44-4 | C12H18Ca3O18 | 570.492 g/mol
Catalog number A14828.22
also known as A14828-22
Price (JPY)Request A Quote
-
Quantity:
100 g
Chemical Identifiers
CAS5785-44-4
IUPAC Nametricalcium bis(2-hydroxypropane-1,2,3-tricarboxylate) tetrahydrate
Molecular FormulaC12H18Ca3O18
InChI KeyLNIZKKFWMDARJV-UHFFFAOYSA-H
SMILESO.O.O.O.[Ca++].[Ca++].[Ca++].OC(CC([O-])=O)(CC([O-])=O)C([O-])=O.OC(CC([O-])=O)(CC([O-])=O)C([O-])=O
View more
Calcium citrate tetrahydrate may be used as raw material in the preparation of nanoparticle with the nominal composition Ca0.8 Ba0.2 Ti03 :Pr3+, via sol-gel process. It may be employed as carbon source in a simple and effective carbonization method to prepare porous carbon without any activation process. It is suitable for use in the preparation of bovine serum albumin (BSA) nanoparticles.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Calcium citrate tetrahydrate may be used as raw material in the preparation of nanoparticle with the nominal composition Ca0.8 Ba0.2 Ti03 :Pr3+, via sol-gel process. It may be employed as carbon source in a simple and effective carbonization method to prepare porous carbon without any activation process. It is suitable for use in the preparation of bovine serum albumin (BSA) nanoparticles.
Solubility
Soluble in water and hydrochloric acid.
Notes
Keep container tightly closed. Store away from oxidizing agents.
Calcium citrate tetrahydrate may be used as raw material in the preparation of nanoparticle with the nominal composition Ca0.8 Ba0.2 Ti03 :Pr3+, via sol-gel process. It may be employed as carbon source in a simple and effective carbonization method to prepare porous carbon without any activation process. It is suitable for use in the preparation of bovine serum albumin (BSA) nanoparticles.
Solubility
Soluble in water and hydrochloric acid.
Notes
Keep container tightly closed. Store away from oxidizing agents.
RUO – Research Use Only
General References:
- Seham A.A. Mansour. Thermal decomposition of calcium citrate tetrahydrate. Thermochimica Acta. 1994, 223, (2), 243-256.
- Qing Qing Zhoua.; Xiang Ying Chena.; Bo Wang. An activation-free protocol for preparing porous carbon from calcium citrate and the capacitive performance. Microporous and Mesoporous Materials. 2012, 158, 155-161.