Search
Thermo Scientific Chemicals
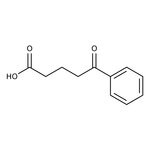
4-Benzoylbutyric acid, 97%
CAS: 1501-05-9 | C11H12O3 | 192.214 g/mol
Catalog number L04013.06
also known as L04013-06
Price (JPY)Request A Quote
-
Quantity:
5 g
Chemical Identifiers
CAS1501-05-9
IUPAC Name5-oxo-5-phenylpentanoic acid
Molecular FormulaC11H12O3
InChI KeySHKWSBAVRQZYLE-UHFFFAOYSA-N
SMILESOC(=O)CCCC(=O)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream to yellow or pale briown
FormPowder or crystalline powder
Assay (Aqueous acid-base Titration)≥96.0 to ≤104.0%
Melting Point (clear melt)122.0-132.0°C
4-Benzoylbutyric acid is an important raw material and intermediate used in organic synthesis, pharmaceuticals, agrochemicals and dyestuff fields.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Benzoylbutyric acid is an important raw material and intermediate used in organic synthesis, pharmaceuticals, agrochemicals and dyestuff fields.
Solubility
Insoluble in water.
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents.
4-Benzoylbutyric acid is an important raw material and intermediate used in organic synthesis, pharmaceuticals, agrochemicals and dyestuff fields.
Solubility
Insoluble in water.
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Steven M. Hutchins.; Kevin T. Chapman. Fischer indole synthesis on a solid support. Tetrahedron Letters. 1996, 37 (28), 4869-4872.
- Jan C. Hummelen.; Brian W. Knight.[ F. LePeq.; Fred Wudl.; Jie Yao.; Charles L. Wilkins. Preparation and Characterization of Fulleroid and Methanofullerene Derivatives. J. Org. Chem. 1995, 60 (3), 532-538.