Written by Nora Marbaniang, Raghul Mathivanan & Amir Khan
What are GPCRs?
G (guanine nucleotide-binding) Protein-Coupled Receptors (GPCRs) are the largest family of cell signaling receptors, with over 800 members (~4%) encoded in the human genome. GPCRs are seven pass-transmembrane proteins that recognize diverse extracellular ligands, such as biogenic amines, lipids, peptides, proteins, ions, and photons. Upon activation by a ligand, GPCRs undergo a conformational change to bind the G-protein and promote exchange of GTP instead of GDP. The heterotrimeric (α, β, γ subunits) G-protein dissociates into βγ and GTP-bound α subunits to mediate a downstream signal cascade and biological effects. GPCR signaling is most common in response to hormones and neurotransmitters and play important roles in vision, olfaction, and taste (1).
Mutations in GPCRs cause more than 30 different human diseases. Not surprisingly, GPCRs are amongst the most exploited drug targets – approximately 30-40% of drugs prescribed for heart failure, hypertension, diabetes, prostate cancer, and bronchial asthma target GPCRs. Recently, GPCRs function and malfunction have been increasingly implicated in cancers and are potential biomarkers for a number of cancers and immune system disorders.
Challenges in GPCR research
The unique biology of GPCRs makes them notoriously difficult to work with (2). Extraction of these highly hydrophobic receptors from the cell membrane into solution without compromising the structural and functional integrity is technically challenging. Inherently, these large transmembrane proteins are poorly expressed compared to other soluble proteins and overexpression often results in failure with localization to an inactive form in the inclusion bodies.
Anti-GPCR antibodies are important research tools and are used in understanding GPCR’s role in diseases such as cancer, infection, inflammation, and cardiovascular disease. However, raising high-quality, specific antibodies against GPCRs is no easy task (2).In addition to the extraction and purification challenges described above, the design of immunogenic antigens is restricted to exposed, hydrophilic regions of the GPCR. Additionally, a single GPCR can exhibit cellular heterogeneity in structure and ligand-binding activities presenting additional challenges in the development of functional anti-GPCR antibodies.
GPCR antibody development
The antibody development team at Thermo Fisher Scientific has developed rabbit recombinant monoclonal and polyclonal antibodies against several GPCR targets by employing a proprietary antibody development platform. Our GPCR antibody development efforts include the usage of specific strategies to obtain immunogens, such as peptides or short protein fragments, and the utilization of glycosylated residues on GCPRs for their enrichment and antibody screening. Once developed, antibody specificity is established using gene knockdown, receptor internalization on cell treatment, and relative expression in different tissues.
A selected list of GPCR antibodies (with advanced verification or multiple references) present in our portfolio is presented in Table 1.
| GPCR Target | Product # |
| DRD1 | MA1-46024 |
| GPR68 | 702595 |
| GHSR | 720278, PA5-28752 |
| GABBR2 | 703357 |
| BDKRB2 | 711638 |
| CRHR1 | 702611 |
| CALCRL | 703811 |
| GPR98 | 703513 |
| GPR30 | PA5-77396, 703480 |
| CCR5 | 12-1951-82, 14-1957-82 |
| CXCR4 | PA3-305, 35-8800, 17-9991-82 |
| ADRA1A | PA1-047 |
| CXCR7 | PA5-27077, PA3-069 |
Table 1. GPCR Targets and available, verified antibodies
In the following sections we highlight findings from a research publication (3), Herzig et al. 2019 ‘Comprehensive assessment of GPR68 expression in normal and neoplastic human tissues using a novel rabbit monoclonal antibody’ (PMID: 31652823), using a GPR68 antibody (Product # 702595)developed using the above-mentioned strategies.
GPR68 recombinant monoclonal antibody for basic research and pathological examinations
GPR68 or ovarian cancer G Protein coupled Receptor 1 (OGR1) is a member of the proton sensing GPCR family. The protein is active at a slightly acidic pH, 6.8, but inactive at pH 7.8. At acidic pH, four histidines in the extracellular loop are protonated resulting in disruption of H-bonds and activation of the protein. Apart from pH, ogerin and benzodiazepine lorazepam act as its chemical activators. GPR68 is reported to play major roles in inflammation, ischaemia, and tumorigenesis. These conditions are characterized by pH changes in the microenvironment.
Contradictory reports exist on the role of GPR68 in tumorigenesis. In cancer cell lines, increased GPR68 expression results in reduced cell proliferation and migration, thus, suggesting tumor suppressor roles. However, GPR68 deficiency in mouse cancer model experiments favored tumor reduction indicating tumor promoter functions. Further investigation is crucial to verify if GPR68 impact varies depending on the tumor cell type, microenvironment, and differences in primary tumors and metastases.
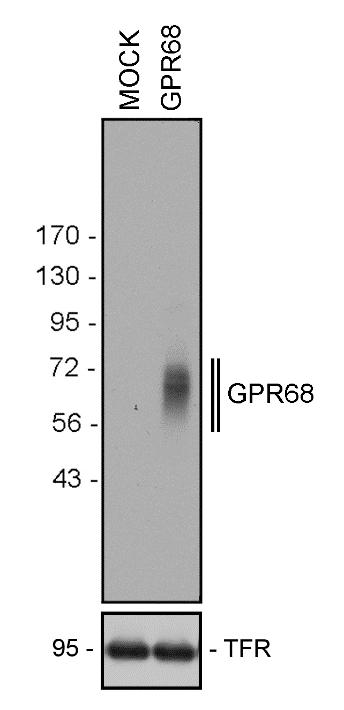
A major limitation of studying GPR68 in tissues as opposed to cancer cell lines is the unavailability of a specific monoclonal antibody with minimal background staining. To develop such a reagent, we used the carboxyl-terminal tail of human GPR68 as an epitope to generate a rabbit recombinant monoclonal antibody (GPR68 Recombinant Rabbit Monoclonal Antibody, 16H23L16, Product # 702595) that could be effectively used for three applications, namely western blot (figure 1A), immunocytochemistry, and immunofluorescence double-labelling of normal and neoplastic tissue sections. Specificity of the antibody could be verified by genetic knockdown using siRNA, resulting in a reduction in signal in western blotting (3).
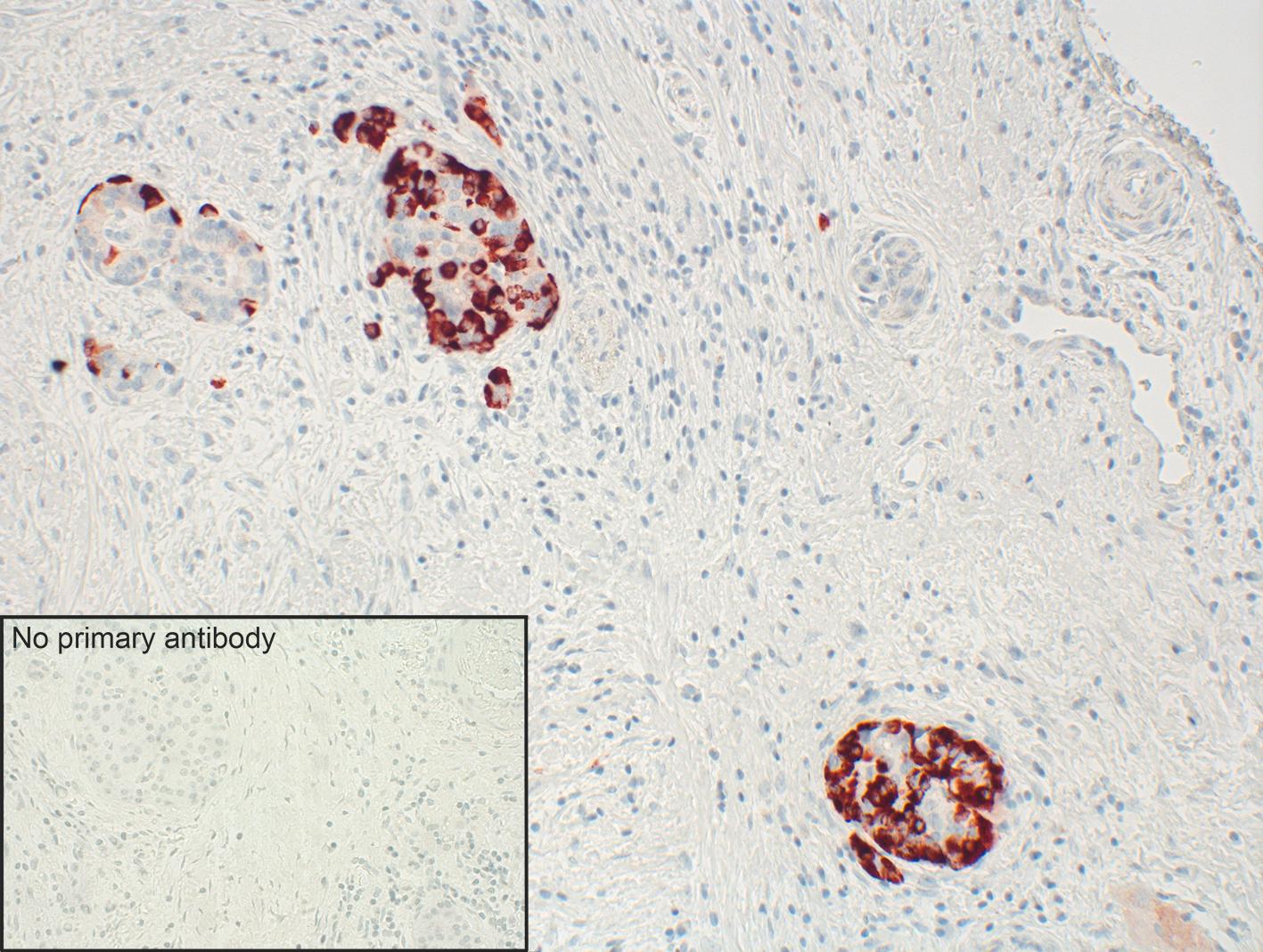
In immunofluorescence, clear cytoplasmic staining could be observed at pH 6.8 or 7.4 and when the lorazepam treatment was used. However, this changed to plasma membrane staining at pH 7.8, mirroring GPR68 biology (3). Immunohistochemistry of normal tissues distinctly revealed GPR68 presence in glucagon- producing alpha cells of pancreas (figure 1B), neuroendocrine cells of the intestinal tract, gastric glands, granulocytes, macrophages, and muscle layers of blood vessels. (3).
A. B.
Figure 1. A Western blot analysis of whole-cell preparations from mock- or stably GPR68-transfected HEK-293 cells using GPR68 Recombinant Rabbit Monoclonal Antibody (16H23L16) (Product # 702595, 1:500 dilution). B. Strong staining of distinct cells in pancreatic islets was observed in the human pancreas tested using the same recombinant rabbit monoclonal antibody in immunohistochemistry. Inset shows control tissue where no primary antibody was added.
To decipher GPR68 roles in cancer and its presence in human tumor samples, Herzig et al assessed GPR68 in a broad panel of tumor tissues. Based on a calculated ‘immune reactive score’ the most prominent antibody staining was attributed in pancreatic and neuroendocrine tumors. The staining results were correlated with clinical data, such as grading, staging, glucagon, or insulin secretion, and patient overall survival. Higher GPR68 expression was noted in well-differentiated tumors compared to the expression in highly aggressive neoplasms and correlated with higher survival rate. This suggests that in neuroendocrine tumors, GPR68 may act as a tumor suppressor (3).
The development of a specific GPR68 antibody that works in a variety of applications is incredibly useful. Research on GPR68 has generated a wealth of information on a GPCR that until a few years ago was characterized as an orphan receptor with unknown endogenous ligands, biologicalfunctions, and clinical relevance.
Additional Antibody Blogs
Let’s get ‘specific’ about the TNFR pathway!
DIY Neurons for antibody validation
Translate to Invitrogen antibodies for your ribosomal protein research!
Drivers of the Chromosomal Passenger Complex
PRMTs: Role in epigenetic regulation
Using Blockers to Unlock Secretory Proteins
CRISPR technology applied to antibody validation to accelerate neurobiology research
Specific and neutralizing recombinant antibodies to SARS-CoV-2
References:
1) Weis, W. I., & Kobilka B. K. (2018). The molecular basis of G protein-coupled receptor activation. Annu. Rev. Biochem. 87:897–919
2) Jo, M., & Jung, S. T. (2016). Engineering therapeutic antibodies targeting G-protein – coupled receptors. Experimental & Molecular Medicine, 48(2), e207-9
3) Herzig, M., Dasgupta, P., Kaemmerer, D., Sänger, J., Evert, K., Schulz, S., & Lupp, A.(2019). Comprehensive assessment of GPR68 expression in normal and neoplastic human tissues using a novel rabbit monoclonal antibody. Int. J. Mol. Sci. 20(21), 5261




Leave a Reply