You would be hard-pressed not to find a high-performance liquid chromatography (HPLC) instrument in today’s analytical laboratory, given its critical role in a range of fields, from pharmaceutical to food and beverage, manufacturing and environmental safety.
This blog post explains what HPLC is, how it works and the different techniques liquid chromatographs use to purify mixtures.
 What is HPLC?
What is HPLC?
HPLC is a broad analytical chemistry technique used to separate, identify and quantify compounds in a chemical mixture. These separations utilize the pressure-driven flow of a mobile phase through a column packed with a stationary phase.
The mobile phase carries a liquid sample through the column to the detector, and compounds — or analytes — separate due to varying degrees of interaction with the stationary phase.
A detector measures the analytes after elution off the column, and a chromatography data system (CDS) translates the detected signal.
The translated data output of an HPLC analysis is called a chromatogram, where the x-axis shows time and the y-axis is a specific signal generated by the detector.
 Figure 1. Example of an HPLC chromatogram.
Figure 1. Example of an HPLC chromatogram.
How does HPLC work?
An HPLC instrument generally has four major hardware components: a pump, autosampler, column and detector. Additional elements include solvents and a CDS package plus connective capillaries and tubing to allow the continuous flow of the mobile phase and sample through the system.
Every HPLC analysis includes the following steps:
- Mobile phase begins to flow — The pump pushes the eluents through the system at a specified flow rate.
- Sample injection — After injection into the mobile phase, the sample travels with the mobile phase from the injection point to the head of the column.
- Compound separation — Physical separation of the compounds happens on the column stationary phase. After elution from the column, the separated sample components travel to the detector.
- Analyte detection — Detection of specified analytes based on an electrical signal generated by specific properties.
- Chromatogram generation — Translation of the detected analyte signal by the CDS into a chromatogram of analyte signal versus time.
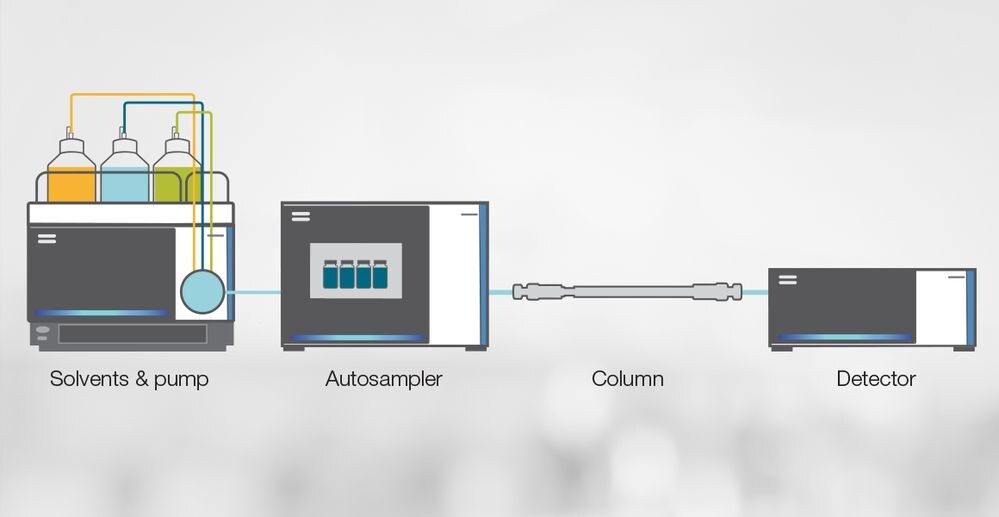 Figure 2: HPLC instrument diagram.
Figure 2: HPLC instrument diagram.
What factors affect HPLC separations?
Many factors like the mobile phase composition, column chemistry, and temperature can influence HPLC separations. Successful separation only occurs if the analytes have differing affinities for the column, so selecting the appropriate stationary phase for your compounds is crucial.
The main factors influencing the overall separation process are:
- Physiochemical properties of the analyte, such as size, charge, polarity and volatility
- Physiochemical properties of the stationary phase, such as polarity, charge and viscosity
- Physiochemical properties of the mobile phase used and interaction with the analyte and stationary phases
Isocratic versus gradient separations
All HPLC separations are carried out in one of two modes — isocratic or gradient.
- Isocratic methodsseparatebyusing a consistent eluent composition duringanalysis, like 100 percent acetonitrile or a 50:50 mixture of acetonitrile to water.
- Gradient methods include a change in the mobile phase composition across a separation. These methods often employ two solvents, called A and B. The run will begin with a certain percentage of A to B, like 60 percent water to 40 percent acetonitrile, for instance, followed by a percentage change throughout a separation.
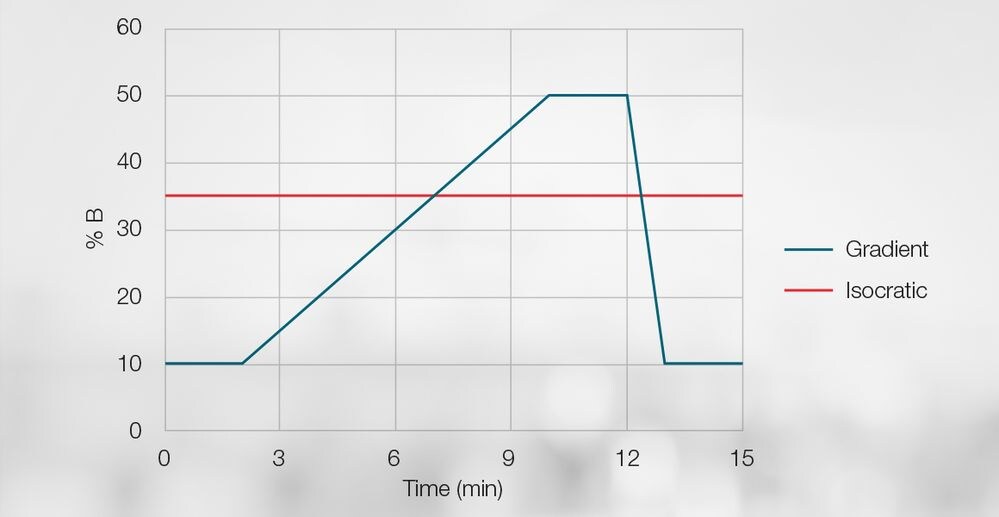 Figure 3. Depiction of isocratic versus gradient elution.
Figure 3. Depiction of isocratic versus gradient elution.
Types of liquid chromatography techniques
Given the vast number of compounds and structural diversity of potential analytes, HPLC is rarely a one-size-fits-all approach. From nano to preparative scale separations, here is a list of the most common types of HPLC techniques and when to apply each.
| LC technique | How it works | Applications |
| High-performance liquid chromatography | HPLCis run at pressures below 700 bar with flow rates between 1 – 2 mL/min | Quality assurance/quality control of small and large molecules in pharmaceuticals, industrial chemicals, and food safety |
| Ultra-high-performance liquid chromatography | UHPLC uses pressures beyond 1000 bar and flow rates from0.2 – 0.7 mL/min. Higher pressure ranges give better resolution and sensitivity, higher throughput, and less solvent usage than standard HPLC systems | Commonly applied in research and development labs and pharma and biopharma fields for the development and characterization of small molecule drugs, peptides, and antibodies |
| Liquid chromatography-mass spectrometry | Liquid chromatography-mass spectrometry (LC-MS) utilizes a mass spectrometer instead of the traditional optical detector like a UV-Vis detector | Mostly used for peptide and protein analysis |
| Low-flow liquid chromatography | Low-flow liquid chromatography encompasses nano-, micro-, and capillary-flow ranges spanning from low nL/min to about 50 µL/min. This technique increases sensitivity due to the associated decrease in column inner diameter, leading to less dilution of analyte bands | Ideal for high-sensitivity measurements of molecules in complex biological matrices where analyte concentrations can span several orders of magnitude |
| Preparative liquid chromatography | Preparative LC techniques involvecollecting fractionated eluentinto discrete sample containers to isolate one or more analytes in order to purify main components or segregate impurities for further investigations | Various applications like large scale purifications of drugs or smaller-scale for improving product yields or isolating pure compounds |
Multichannel LC Systems
Multichannel LC systems use multiple flow paths to help chromatographers perform complex and/or parallel separation processes. The benefits of multichannel systems can include higher sample throughput, better resolution of complex samples, and enhanced analyte quantitation
| Multichannel LC technique | How it works | Applications |
| Two-dimensional liquid chromatography | 2D-LC is an advanced separation technique using wo complementary column chemistries in series for a multi-dimensional separation instead of running the sample through one column | Applications of 2D-LC can apply to complex chemical mixtures like vaccines and foods with interfering sample matrices |
| Dual liquid chromatography | Dual LC is a multichannel HPLC method using two separate flow paths in a single system to run two analyses simultaneously | Helpful for situations when you need to increase sample throughput, like analyzing a sample for pesticides and phenol in a single run or performing replicate analyses simultaneously |
| Tandem liquid chromatography | Tandem LC techniques use a second pump and intelligent column switching to maximize detector usage by minimizing downtime from column reconditioning | Best used in lead selection for drug discovery labs to increase sample throughput and maximize detector utilization |
Want to learn more about HPLC analysis?
It’s been incredible to see the evolution of liquid chromatography in real time. From the beginning, separating pigments, to basic liquid chromatography, it was a very manual process that took hours to separate compounds of interest. Today, with modern HPLC and UHPLC instruments, separations are done within minutes, even seconds in some cases.
Additionally, utilizing various detectors such as UV, mass spectrometry and charged aerosol; detecting, identifying and quantifying your compounds is immensely more effortless than ever before.
- Watch: The Evolutionary Journey of HPLC for Thermo Fisher.
- Visit our learning center to learn more about HPLC and access our virtual HPLC instrument product tours.
- And don’t forget to follow our Chromatography & Mass Spectrometry page on LinkedIn.

Elevate Your HPLC Performance with a Modern CDS
You may have the best HPLC system on the market, but to maxi...
Read More
Episode 3: Understanding How Charged Aerosol Detection (CAD) Works
Welcome back to our series on Charged Aerosol Detection (CAD...
Read More
Episode 2: The Benefits of CAD Compared to Other Universal Detectors: ELSD and MALS
Welcome back to our series on Charged Aerosol Detection (CAD...
Read More
Episode 1: The Benefits of Charged Aerosol Detection (CAD) in High-Performance Liquid Chromatography (HPLC)
High-Performance Liquid Chromatography (HPLC) is a cornersto...
Read More