Expressing Recombinant Proteins
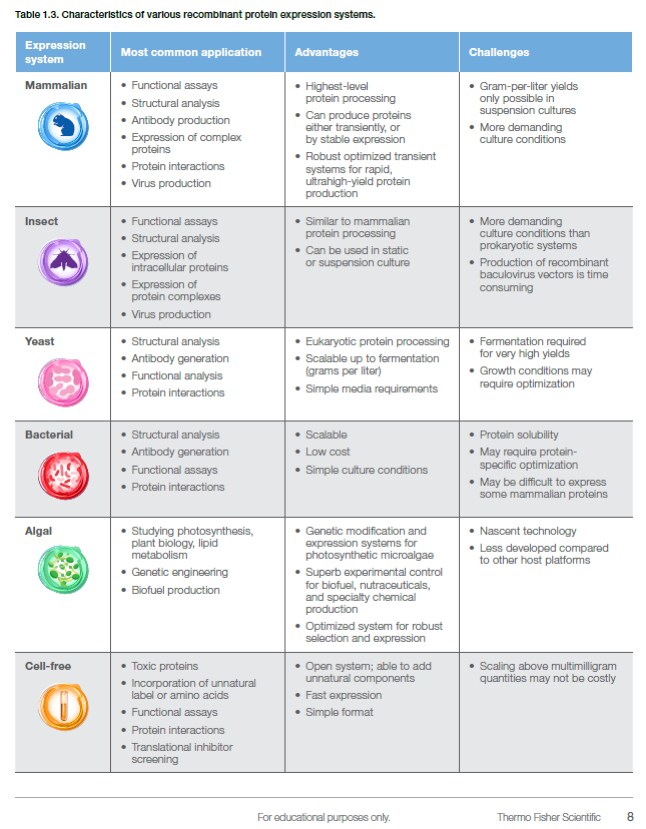
Many scientific research projects are based on investigating transiently or stably expressed recombinant proteins in mammalian, insect, yeast, bacterial, or cell-free systems. Achieving detectable and reliable protein yields in these systems can be challenging, and using the right expression host for your specific application is key to your success. Protein solubility, functionality, purification speed, and expected yield are often crucial factors that help to narrow your choices. While bacterial systems provide high protein yields in the shortest amount of time and mammalian systems are capable of producing proteins with complete post-translational modifications closest to that of humans, an insect expression system is suitable for expressing intracellular proteins and multi-protein complexes. To help you choose the system that best suits your needs, check out Table 1.3. It provides the main characteristics of several expression systems including the most common applications, advantages, and challenges with each system.
Insect Expression Systems
If you decide that insect expression is right for your experiment, you’ll want to look into baculovirus-based methods. Baculovirus expression systems offer high levels of protein expression with simple glycosylation and post-translational modifications, ease of scale-up, and simplified cell growth that can be readily adapted to high-density suspension culture for large-scale protein expression in insect cells. Expression levels up to 900 mg/L can be achieved using the Gibco ExpiSf™ Expression System. This baculovirus-based insect system has been optimized with the Bac-to-Bac™ technology for the generation of baculovirus by site-specific transposition of a shuttle vector in specialized bacterial cells to generate an expression bacmid containing the gene of interest. The bacmid DNA is then transfected into insect cells to generate recombinant baculovirus. Subsequent protein expression in specially adapted Sf9 cells (ExpiSf9™ insect cells) offers several advantages over mammalian cells including a fast and cost-effective method for generating recombinant baculovirus, ability to accommodate very large DNA insert sizes, and a strong promoter ideal for high level expression of recombinant protein.
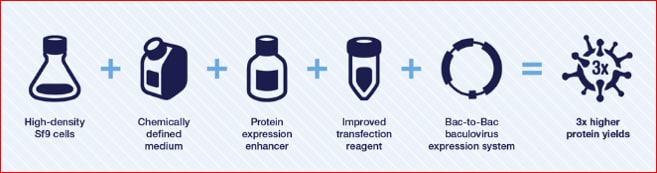
The ExpiSf Expression System components work synergistically for a streamlined workflow and maximum protein yields.
Answers to the Top 5 Insect Expression System FAQs
Insect systems can be challenging to work with, especially in comparison to bacterial systems, and may require you to optimize areas of the workflow to ensure maximum protein yields. Areas of difficulty for less experienced users can include poor insect cell health, difficulty generating and isolating the recombinant bacmid DNA, low viral titers, low transfection efficiency, and low expression yield for some proteins. However, with a few simple tips and tricks under hat, even the most novice user can achieve insect expression success in no time.
To assist you, we’ve summarized some suggested troubleshooting tips for the top 5 commonly encountered scenarios when working with an insect expression system such as the ExpiSf Expression System:
1. I’m getting only a few colonies after transforming the DH10Bac™ cells with the bacmid, and the blue/white color development sometimes isn’t clear.
Tip: Always use fresh agar plates to ensure the antibiotics are fresh.
Tip: Be sure to use SOC medium during the 4 hour transformation recovery step with shaking at 37°C. Substituting with less-rich LB medium can negatively affect bacterial health.
Tip: Your gene insert may be unstable. Try incubating at 30°C with shaking for 6 hours during the transformation recovery step instead of 37°C for 4 hours.
Tip: If there is poor blue/white colony differentiation, you may need to substitute Bluo-Gal for X-gal, and ensure plates are incubated for at least 48-60 hours for proper color development with 20-60 µg/ml IPTG inducer.
2. I’m having problems producing P0 virus by transfection.
Tip: The complex mixing technique should be gentle as the bacmid DNA/lipid complexes are sensitive to shearing forces.
Tip: The quality of bacmid DNA is important for getting high P0 virus titer. Make sure to verify the general integrity of bacmid by gel electrophoresis, and verify your insert by PCR. To avoid shearing of the large >130 kb bacmid construct, handle very gently in all steps and do not vortex or vigorously mix the bacmid DNA. Be very gentle with the DH10Bac cells as well. Avoid freeze/thawing the bacmid and store it at 4°C for short term usage within 2 weeks.
Tip: For best results, allow ExpiSf9 cells to recover from thaw for 3 passages before transfection. Also, we recommend thawing a new vial of cells after they have been in culture for 25-30 passages. Unlike other insect cells, ExpiSf9 cells are adapted to high-density suspension culture, and therefore, do not reach log phase until 4×106 cells/mL. Passage cells when they reach 5×106 cells/mL (density between 5×106 cells/ml to 10×106 cells/ml in suspension culture). We recommend using ExpiSf CD Medium without any supplementation or antibiotic for optimal transfection efficiency with the ExpiFectamine Sf Transfection Reagent.
3. My ExpiSf9 cells are infected and dying but I have very little to no protein expression.
Tip: Cells may have been harvested too late, allowing the protein to degrade. Most proteins can be harvested at day 3-4, but ideally you should perform a time course experiment harvesting cells at different time points: 48 h, 60 h, 72 h, 96 h, etc. Remember that baculovirus is a lytic virus, so your infected cells will eventually lyse.
Tip: The MOI may be too high. Perform a viral titer assay to accurately determine the concentration of the viral stock. Then try different MOIs: 1, 5, and 10 often work well.
Tip: The cell density may have been too low or too high for optimal baculovirus infection and protein expression. ExpiSf9 cells should be seeded at 5×106 cells/mL and immediately treated with ExpiSf Enhancer. 18-24 h later, cells should be at a density of 5×106 to 7×106 cells/ml, at which point they are ready to be infected with virus. Cell density >8×106 cells/ml at time of infection may lead to poor infectivity and protein expression.
Tip: Protein may not be in the expected fraction. If looking for secreted protein, be sure to check the cell pellet as well because secretion efficiency will almost never be 100%. Also, remember that cells will lyse during baculovirus infection so intracellular proteins can often be found in both the cell lysate and the supernatant.
Tip: There may be an unexpected frame shift, recombination error, or premature stop codon present. Confirm by sequencing that your gene of interest was inserted correctly into the bacmid.
4. My ExpiSf9 cells do not show any clear signs of infection after 72 hours and I have very little to no protein expression.
Tip: The MOI may have been too low. Perform a viral titer assay to accurately determine the amount of virus in your stock. Then try different MOIs: 5, 10, or higher.
Tip: The cell density may be too high or cells are too old. For optimal protein expression, ExpiSf9 cells in suspension culture at the time of infection should be at a passage number of less than 30.
5. I obtained good expression in small scale but lost expression in large scale.
Tip: You may need to adjust some of your parameters slightly at larger scale. Try different MOIs and/or different harvesting times. Try fine tuning the harvesting time +/- 6 hours while keeping the MOI constant.
Didn’t find your answer here in the top 5 insect expression system FAQs? Find answers to more questions in our ExpiSf Expression System FAQs and insect expression support center.
 ExpiSf Expression System Offers 3X Higher Protein Yields Than Previous Insect Systems in a Shorter Time.
ExpiSf Expression System Offers 3X Higher Protein Yields Than Previous Insect Systems in a Shorter Time.
For the highest protein yields from an insect expression system, you’ll want to try Thermo Fisher’s ExpiSf Expression System. It’s the first insect expression system that includes both a chemically defined medium and protein expression enhancer that outperforms existing platforms with 3X higher protein yields. The fully integrated ExpiSf Expression System Starter Kit includes everything you’ll need, including optimized ExpiSf™ CD Medium, ExpiSf9™ cells, ExpiSf™ Enhancer, ExpiFectamine™ Sf transfection reagent, pFastBac™ vector, DH10Bac™ competent cells, and a streamlined workflow to deliver maximum protein yields in as little as 6 days.
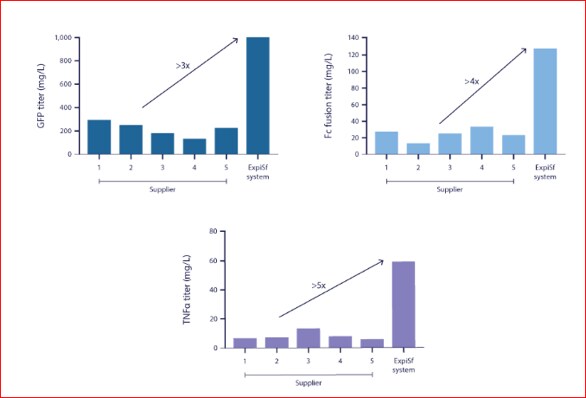
The ExpiSf system outperforms existing insect platforms. In performance tests against other suppliers’ media, the ExpiSf system demonstrated greater protein expression, delivering 3x more protein.
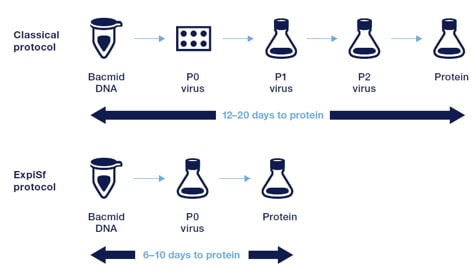
The ExpiSf system delivers protein in half the time. Bacmid DNA transfection in suspension using the ExpiSf system allows for efficient baculovirus generation without the need for virus amplification, delivering protein in as few as 6 days.
For more information on the ExpiSf Expression System, visit www.thermofisher.com/expisf
 New to Insect Protein Expression?
New to Insect Protein Expression?
Register for the SmartStart™ Training for the ExpiSf Expression System through the LabCoat Live™ learning environment
Getting started with an unfamiliar application or a new product can often present challenges. Receiving instruction from a scientist who is highly experienced and very familiar with the ExpiSf Expression System can help enable your success.
LabCoat Live is a unique learning environment that allows you to gain hands-on experience in your lab by providing the necessary reagents to complete the protocol(s) and offering lectures through a flexible and affordable online environment. Our experienced application scientists will guide you step-by-step, via a series of live online lectures, as you complete a self-paced hands-on experiment in your own lab. Upon completion of the course, you’ll have access to the online tools, discussion forum, and classroom companion which includes downloadable notes and protocols, support videos, and other supplemental information for a few months. Because class size is limited and you conduct the protocol yourself, you’ll benefit from the LabCoat Live learning environment by receiving individualized instruction and hands-on practice that will increase your knowledge retention.
In the LabCoat Live: SmartStart Training for the ExpiSf Expression System, you’ll learn the fundamentals of baculovirus generation and protein expression using the ExpiSf Expression System Starter Kit. The course covers detailed reviews of the unique system components and the key workflow steps where optimal techniques will maximize results. The course includes the starter kit, five live online lectures, access to videos and support guides, and a dedicated forum to get your questions answered quickly. After attending the course, you’ll be able to apply best practices to efficiently clone and isolate bacmids, generate baculovirus stock, and express maximum protein from ExpiSf9 cells.
Is an insect expression system right for your protein? For more information on insect protein expression systems, visit www.thermofisher.com/insectexpression
For Research Use Only. Not for use in diagnostic procedures.
Leave a Reply