Search Thermo Fisher Scientific
Thermo Scientific Chemicals
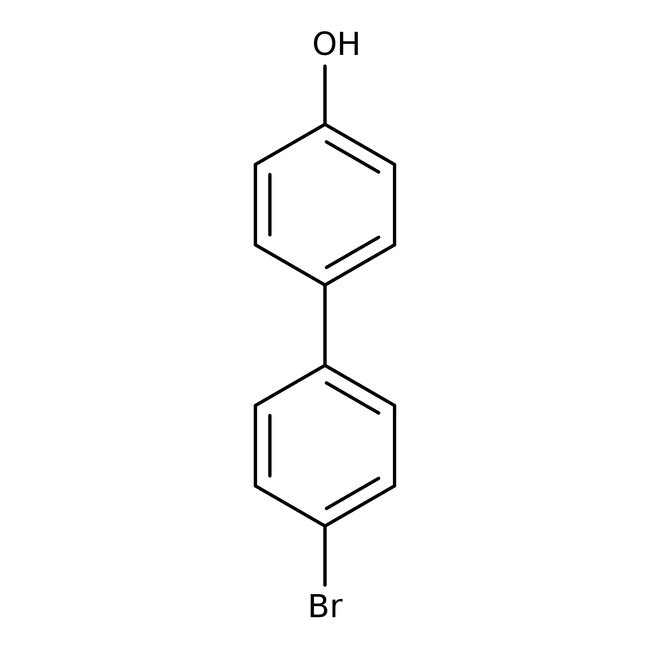
4-Bromo-4'-hydroxybiphenyl, 98%, Thermo Scientific Chemicals
Catalog number: A10819.22
100 g, Each


Thermo Scientific Chemicals
4-Bromo-4'-hydroxybiphenyl, 98%, Thermo Scientific Chemicals
Catalog number: A10819.22
100 g, Each
Quantity
Catalog number: A10819.22
also known as A10819-22
Price (USD)
Price: 716.00
Online price: 619.65
Your price:
Quantity
-
Chemical Identifiers
CAS
29558-77-8
IUPAC Name
4'-bromo-[1,1'-biphenyl]-4-ol
Molecular Formula
C12H9BrO
InChI Key
ARUBXNBYMCVENE-UHFFFAOYSA-N
SMILES
OC1=CC=C(C=C1)C1=CC=C(Br)C=C1
Specifications
Assay (GC)
≥97.5%
Appearance (Color)
White to cream
Assay (Silylated GC)
≥97.5%
Form
Powder
Melting Point (clear melt)
164.0-170.0?C
Description
A biphenyl starting material. 4-Bromo-(1,1-biphenyl)-4-ol is a useful intermediate. Vinylation of 4-bromo-4-hydroxybiphenyl and ethyl acrylate using Pd (OAc) 2/PPh 3 catalyst was studied. Ethyl 4-(4-hydroxyphenyl) cinnamate was formed as the vinylation product, while, 4-hydroxybiphenyl and ethyl cinnamate were formed as side products. Preparation of 4-cyano-4'-hydroxybiphenyl This was prepared from 4-bromo-4'-benzenesulphonyloxybiphenyl by first hydrolysing it to 4-bromo-4-hydroxybiphenyl using sodium hydroxide dissolved in a mixture of water and dioxan. The syntheses of the Nanocomposite dendrimers based on cyclic phosphazene cores: Amorphous materials, were accomplished by following a modified literature procedure by reacting phosphonitrilic chloride trimer with 4-bromophenol or 4-bromo-4-hydroxybiphenyl, respectively, in the presence of K 2 CO 3 in tetrahydrofuran (THF).
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
A biphenyl starting material. 4′-Bromo-(1,1′-biphenyl)-4-ol is a useful intermediate. Vinylation of 4-bromo-4′-hydroxybiphenyl and ethyl acrylate using Pd (OAc) 2/PPh 3 catalyst was studied. Ethyl 4-(4′-hydroxyphenyl) cinnamate was formed as the vinylation product, while, 4-hydroxybiphenyl and ethyl cinnamate were formed as side products. Preparation of 4-cyano-4′-hydroxybiphenyl This was prepared from 4-bromo-4′-benzenesulphonyloxybiphenyl by first hydrolysing it to 4-bromo-4-hydroxybiphenyl using sodium hydroxide dissolved in a mixture of water and dioxan. The syntheses of the Nanocomposite dendrimers based on cyclic phosphazene cores: Amorphous materials, were accomplished by following a modified literature procedure by reacting phosphonitrilic chloride trimer with 4-bromophenol or 4-bromo-4′-hydroxybiphenyl, respectively, in the presence of K 2 CO 3 in tetrahydrofuran (THF).
Solubility
Soluble in water (partly), and methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
A biphenyl starting material. 4′-Bromo-(1,1′-biphenyl)-4-ol is a useful intermediate. Vinylation of 4-bromo-4′-hydroxybiphenyl and ethyl acrylate using Pd (OAc) 2/PPh 3 catalyst was studied. Ethyl 4-(4′-hydroxyphenyl) cinnamate was formed as the vinylation product, while, 4-hydroxybiphenyl and ethyl cinnamate were formed as side products. Preparation of 4-cyano-4′-hydroxybiphenyl This was prepared from 4-bromo-4′-benzenesulphonyloxybiphenyl by first hydrolysing it to 4-bromo-4-hydroxybiphenyl using sodium hydroxide dissolved in a mixture of water and dioxan. The syntheses of the Nanocomposite dendrimers based on cyclic phosphazene cores: Amorphous materials, were accomplished by following a modified literature procedure by reacting phosphonitrilic chloride trimer with 4-bromophenol or 4-bromo-4′-hydroxybiphenyl, respectively, in the presence of K 2 CO 3 in tetrahydrofuran (THF).
Solubility
Soluble in water (partly), and methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text