Search
Thermo Scientific Chemicals
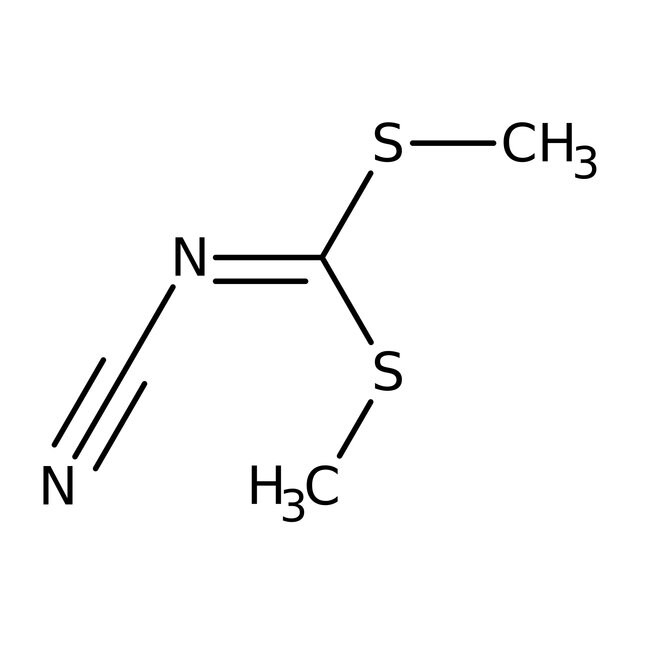
Dimethyl cyanodithioiminocarbonate, 95%
CAS: 10191-60-3 | C4H6N2S2 | 146.226 g/mol
化学物質識別子
CAS10191-60-3
IUPAC Name[bis(methylsulfanyl)methylidene](cyano)amine
Molecular FormulaC4H6N2S2
InChI KeyIULFXBLVJIPESI-UHFFFAOYSA-N
SMILESCSC(SC)=NC#N
さらに表示
仕様 スペックシート
スペックシート
Appearance (Color)White to yellow or pale brown
FormPowder and/or Lumps
Assay (GC)>94.0%
Water Content (Karl Fischer Titration)<2.0%
Dimethyl cyanodithioiminocarbonate has been used in the synthesis of 4-methylthiopyrazolo[1,5-a]-1,3,5-triazines, methylsulfanylpyrimidines, cyanoguanidines and N-aryl-6-methylsulfanyl-4-oxopyrimidine-5-carbonitriles. It has been also used in the synthesis of methylsulfanyl derivatives of azoloazines and azoloazoles.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Dimethyl cyanodithioiminocarbonate has been used in the synthesis of 4-methylthiopyrazolo[1,5-a]-1,3,5-triazines, methylsulfanylpyrimidines, cyanoguanidines and N-aryl-6-methylsulfanyl-4-oxopyrimidine-5-carbonitriles. It has been also used in the synthesis of methylsulfanyl derivatives of azoloazines and azoloazoles.
Solubility
Slightly soluble in methanol.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
Dimethyl cyanodithioiminocarbonate has been used in the synthesis of 4-methylthiopyrazolo[1,5-a]-1,3,5-triazines, methylsulfanylpyrimidines, cyanoguanidines and N-aryl-6-methylsulfanyl-4-oxopyrimidine-5-carbonitriles. It has been also used in the synthesis of methylsulfanyl derivatives of azoloazines and azoloazoles.
Solubility
Slightly soluble in methanol.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Yong-Qian Wu; Sean K.Hamilton; Douglas E.Wilkinson; Gregory S.Hamilton. Direct synthesis of guanidines using Di(imidazole-1-yl)methanimine. J. Org. Chem. 2002, 67,(21), 7553-7556.
- Aiguo Zhang; Hartmut Kaiser; Peter Maienfisch; John E.Casida. Insect nicotinic acetylcholine receptor: Conserved neonicotinoid specificity of [3H]imidacloprid binding site. Journal of Neurochemistry. 2000, 75,(3), 1294-1303.