Search
Thermo Scientific Chemicals
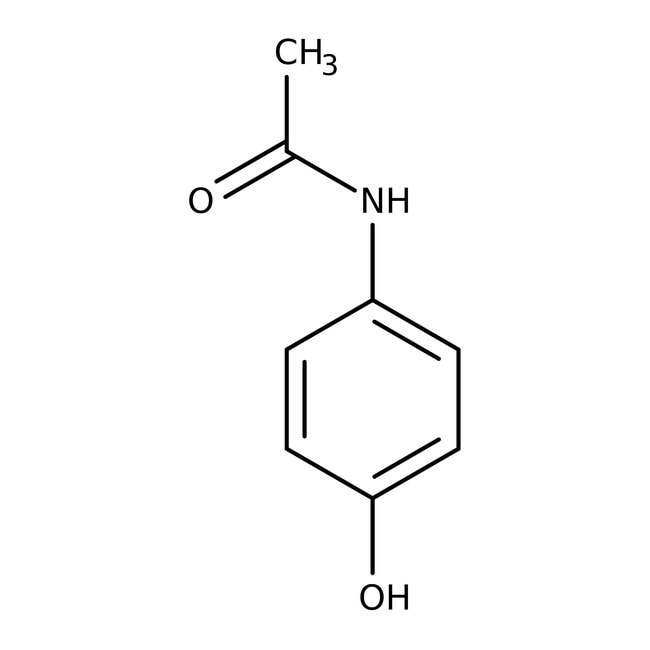
4-Acetamidophenol, 98%
CAS: 103-90-2 | C8H9NO2 | 151.17 g/mol
化学物質識別子
CAS103-90-2
IUPAC NameN-(4-hydroxyphenyl)acetamide
Molecular FormulaC8H9NO2
InChI KeyRZVAJINKPMORJF-UHFFFAOYSA-N
SMILESCC(=O)NC1=CC=C(O)C=C1
さらに表示
仕様 スペックシート
スペックシート
FormCrystals or powder or crystalline powder
Melting Point (clear melt)167.0-173.0?C
Appearance (Color)White
Assay (HPLC)≥97.5%
Identification (FTIR)Conforms
Cyclooxygenase inhibitor and widely used analgesic. 4-Acetamidophenol formulated pharmaceutical products are used as antiinfectant, analgesic, anti rheumatic and antipyretic. It is used as an intermediate in organic synthesis, hydrogen peroxide stabilizer and photographic chemicals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Cyclooxygenase inhibitor and widely used analgesic. 4-Acetamidophenol formulated pharmaceutical products are used as antiinfectant, analgesic, anti rheumatic and antipyretic. It is used as an intermediate in organic synthesis, hydrogen peroxide stabilizer and photographic chemicals.
Solubility
Soluble in alcohol, dimethylformamide, ethylene dichloride, acetone and ethyl acetate. Slightly soluble in water and ether.
Notes
Incompatible with strong oxidizing agents, acids and alkali metals.
Cyclooxygenase inhibitor and widely used analgesic. 4-Acetamidophenol formulated pharmaceutical products are used as antiinfectant, analgesic, anti rheumatic and antipyretic. It is used as an intermediate in organic synthesis, hydrogen peroxide stabilizer and photographic chemicals.
Solubility
Soluble in alcohol, dimethylformamide, ethylene dichloride, acetone and ethyl acetate. Slightly soluble in water and ether.
Notes
Incompatible with strong oxidizing agents, acids and alkali metals.
RUO – Research Use Only
General References:
- Pintal, M.; Kryczka, B.; Marsura, A.; Porwanski, S. Synthesis of bis-cellobiose and bis-glucose derivatives of azacrown macrocycles as hosts in complexes with acetylsalicylic acid and 4-acetamidophenol. Carbohydr. Res. 2014, 386, 18-22.
- Albert, A.; Engelhard, C. Chemometric optimization of a low-temperature plasma source design for ambient desorption/ionization mass spectrometry. Spectrochim. Acta, Part B 2015, 105, 109-115.