Search
Thermo Scientific Chemicals
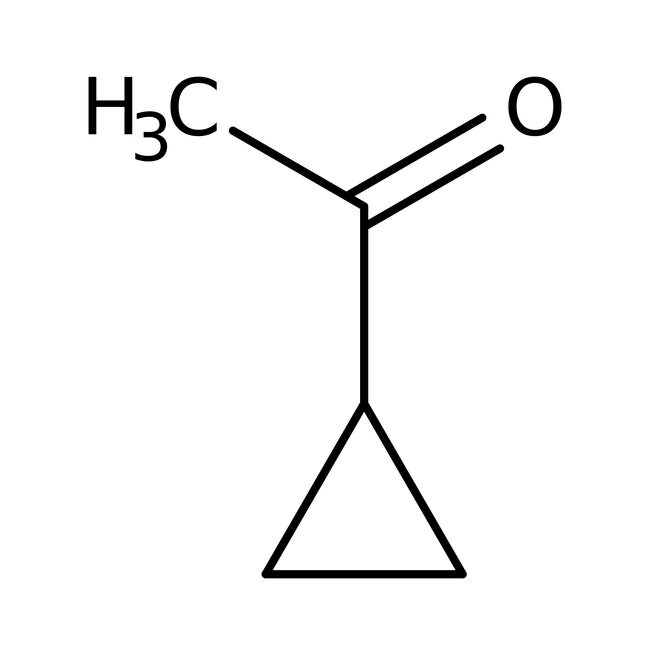
Cyclopropyl methyl ketone, 99%
CAS: 765-43-5 | C5H8O | 84.118 g/mol
化学物質識別子
CAS765-43-5
IUPAC Name1-cyclopropylethan-1-one
Molecular FormulaC5H8O
InChI KeyHVCFCNAITDHQFX-UHFFFAOYSA-N
SMILESCC(=O)C1CC1
さらに表示
仕様 スペックシート
スペックシート
Identification (FTIR)Conforms
Appearance (Color)Clear colorless
FormLiquid
Assay (GC)≥98.5%
Refractive Index1.4230-1.4260 @ 20?C
Cyclopropyl methyl ketone was used in the preparation of homoallylic alcohols. Acetylcyclopropane is used as an intermediate for the manufacturing pharmaceuticals, agrochemicals and other organic compounds.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Cyclopropyl methyl ketone was used in the preparation of homoallylic alcohols. Acetylcyclopropane is used as an intermediate for the manufacturing pharmaceuticals, agrochemicals and other organic compounds.
Solubility
Fully miscible in water.
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
Cyclopropyl methyl ketone was used in the preparation of homoallylic alcohols. Acetylcyclopropane is used as an intermediate for the manufacturing pharmaceuticals, agrochemicals and other organic compounds.
Solubility
Fully miscible in water.
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
General References:
- L. S. Bartell.; J. P. Guillory.; Andrea T. Parks. Electron Diffraction Study of the Structure and Conformational Behavior of Cyclopropyl Methyl Ketone and Cyclopropanecarboxylic Acid Chloride1a. J. Phys. Chem. 1965, 69, (9), 3043-3048.
- William G. Dauben.; Richard E. Wolf. Transition-state conformations in the reductive opening of cyclopropyl methyl ketones. J. Org. Chem. 1970, 35, (7), 2361-2367.
- Various cyclopropyl ketones have been used in the synthesis of δ-ketonic phosphonium salts which, protected as thioacetals, have found use in the Wittig reaction: Synthesis, 648 (1987):