Search
Thermo Scientific Chemicals
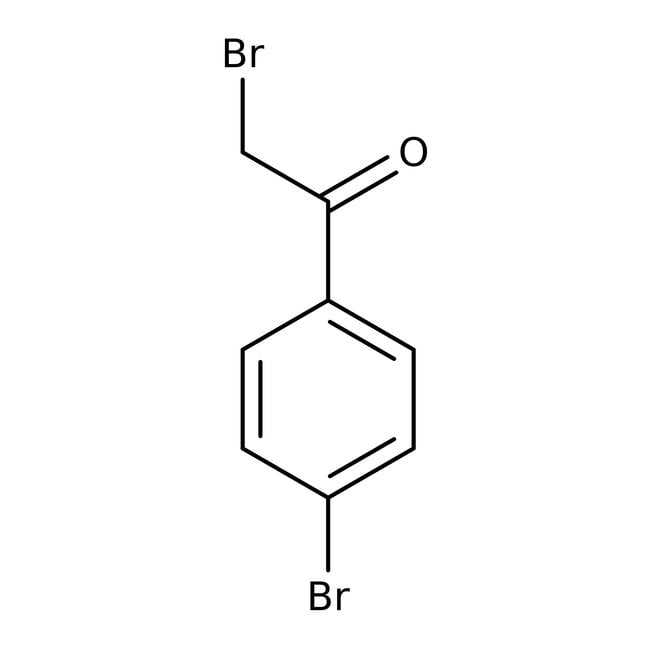
2,4'-Dibromoacetophenone, 98+%
CAS: 99-73-0 | C8H6Br2O | 277.943 g/mol
化学物質識別子
CAS99-73-0
IUPAC Name2-bromo-1-(4-bromophenyl)ethan-1-one
Molecular FormulaC8H6Br2O
InChI KeyFKJSFKCZZIXQIP-UHFFFAOYSA-N
SMILESBrCC(=O)C1=CC=C(Br)C=C1
さらに表示
仕様 スペックシート
スペックシート
FormCrystals or powder or crystalline powder
Assay (GC)≥98.0%
Identification (FTIR)Conforms
Melting Point (clear melt)108.0-114.0?C
Appearance (Color)White to pale cream
2,4'-Dibromoacetophenone is used as a reagent for analysis of fatty acids by HPLC as 4-bromophenacyl esters, for use in the protection of phenols and carboxylic acids. It undergoes condensation reactions with aldehydes in the presence of SnCl2 or SmI3 to afford α,β-unsaturated ketones. It is also useful in the esterification of carboxylic acids.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,4′-Dibromoacetophenone is used as a reagent for analysis of fatty acids by HPLC as 4-bromophenacyl esters, for use in the protection of phenols and carboxylic acids. It undergoes condensation reactions with aldehydes in the presence of SnCl2 or SmI3 to afford α,β-unsaturated ketones. It is also useful in the esterification of carboxylic acids.
Solubility
Soluble in dimethyl sulfoxide (5 mg/ml), methanol (20 mg/ml), toluene and ethanol. Insoluble in water.
Notes
Keep container tightly sealed. Store in refrigerated condition. Incompatible with bases and oxidizing agents.
2,4′-Dibromoacetophenone is used as a reagent for analysis of fatty acids by HPLC as 4-bromophenacyl esters, for use in the protection of phenols and carboxylic acids. It undergoes condensation reactions with aldehydes in the presence of SnCl2 or SmI3 to afford α,β-unsaturated ketones. It is also useful in the esterification of carboxylic acids.
Solubility
Soluble in dimethyl sulfoxide (5 mg/ml), methanol (20 mg/ml), toluene and ethanol. Insoluble in water.
Notes
Keep container tightly sealed. Store in refrigerated condition. Incompatible with bases and oxidizing agents.
RUO – Research Use Only
General References:
- K. T. Potts.; D. R. Choudhury.; T. R. Westby. Bridgehead nitrogen systems. X. Cycloadditions with thiazolium N-ylides. J. Org. Chem. 1976, 41 (2), 187-191.
- Derrick L. J. Clive.; Pierre L. Beaulieu. Dehalogenation of .alpha.-chloro and .alpha.-bromo ketones. Use of sodium O,O-diethyl phosphorotelluroate. J. Org. Chem. 1982, 47 (6), 1124-1126.
- Reagent for analysis of fatty acids by HPLC as 4-bromophenacyl esters: Anal. Chem., 47, 1797, (1975).
- For use in the protection of phenols and carboxylic acids, see: Tetrahedron Lett., 343 (1970).
- The O-methyl oxime has been used to N-alkylate pyridines. The products can be cyclized with triethylamine to give imidazo[1,2-a]pyridines in high yield: Synthesis, 927 (1996).