Search
Thermo Scientific Chemicals
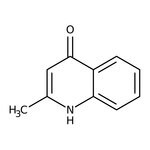
4-Hydroxy-2-methylquinoline, 98+%
CAS: 607-67-0 | C10H9NO | 159.19 g/mol
化学物質識別子
CAS607-67-0
IUPAC Name2-methyl-1,4-dihydroquinolin-4-one
Molecular FormulaC10H9NO
InChI KeyNWINIEGDLHHNLH-UHFFFAOYSA-N
SMILESCC1=CC(=O)C2=CC=CC=C2N1
さらに表示
仕様 スペックシート
スペックシート
Appearance (Color)Pale cream to cream to pale brown
FormPowder
Melting Point (clear melt)230.5-236.5?C
Assay (HPLC)≥98.0%
4-Hydroxy-2-methylquinoline is act as an intermediate in the synthesis of dequalinium chloride and as pharmaceutical intermediate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Hydroxy-2-methylquinoline is act as an intermediate in the synthesis of dequalinium chloride and as pharmaceutical intermediate.
Solubility
Slightly soluble in water.
Notes
Store in cool, dry conditions in a well sealed container. Store away from oxidizing agents.
4-Hydroxy-2-methylquinoline is act as an intermediate in the synthesis of dequalinium chloride and as pharmaceutical intermediate.
Solubility
Slightly soluble in water.
Notes
Store in cool, dry conditions in a well sealed container. Store away from oxidizing agents.
RUO – Research Use Only
General References:
- Thomas J. Lanza; Philippe L. Durette; Thomas Rollins; Salvatore Siciliano; Dana N. Cianciarulo; Sumire V. Kobayashi; Charles G. Caldwell; Martin S. Springer; William K. Hagmann.Substituted 4,6-diaminoquinolines as inhibitors of C5a receptor binding. J. Med. Chem. 1992, 35 (2), 252-258.
- Elise A.Brandenburger Brown; John E Franklin; Eileen Pratt; Eberhard G Trams. Contributions to the pharmacology of quinaldine (uptake and distribution in the shark and comparative studies). Comparative Biochemistry and Physiology Part A: Physiology. 1972, 42 (1), 223-231.
- 4-Hydroxy-2-methylquinolines may be converted to benzo[a]acridines by condensation with aryl aldehydes followed by a photochemical cyclization: Synthesis, 217 (1991).