Search
Thermo Scientific Chemicals
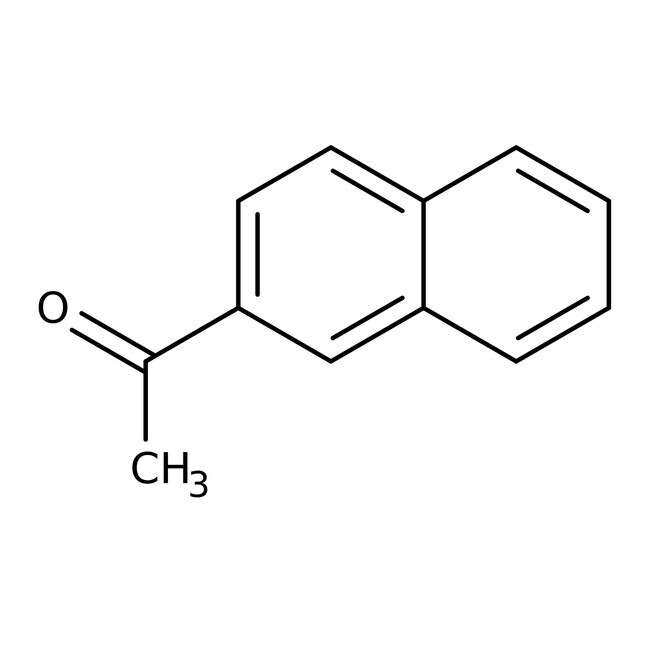
2-Acetylnaphthalene, 99%
CAS: 93-08-3 | C12H10O | 170.211 g/mol
化学物質識別子
CAS93-08-3
IUPAC Name1-(naphthalen-2-yl)ethan-1-one
Molecular FormulaC12H10O
InChI KeyXSAYZAUNJMRRIR-UHFFFAOYSA-N
SMILESCC(=O)C1=CC=C2C=CC=CC2=C1
さらに表示
仕様 スペックシート
スペックシート
Appearance (Color)White to pale yellow
FormCrystals or powder or crystalline powder or lumps or chunks
Assay (GC)≥98.5%
Identification (FTIR)Conforms
Melting Point (clear melt)50.0-57.0?C
2-Acetylnaphthalene is used as a flavoring agent in food. It serves as an intermediate in the preparation of piperidinyl pyrazoles as potent DNA gyrase inhibitors. Further, it is used in air care products, cleaning and furnishing care products. In addition to this, it is used in the synthesis of aromatic enone and dienone analogues of curcumin as angiogenesis inhibitors.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Acetylnaphthalene is used as a flavoring agent in food. It serves as an intermediate in the preparation of piperidinyl pyrazoles as potent DNA gyrase inhibitors. Further, it is used in air care products, cleaning and furnishing care products. In addition to this, it is used in the synthesis of aromatic enone and dienone analogues of curcumin as angiogenesis inhibitors.
Solubility
Soluble in ethanol, acetone and ether. Insoluble in water.
Notes
Incompatible with strong oxidizing agents, strong bases and strong reducing agents.
2-Acetylnaphthalene is used as a flavoring agent in food. It serves as an intermediate in the preparation of piperidinyl pyrazoles as potent DNA gyrase inhibitors. Further, it is used in air care products, cleaning and furnishing care products. In addition to this, it is used in the synthesis of aromatic enone and dienone analogues of curcumin as angiogenesis inhibitors.
Solubility
Soluble in ethanol, acetone and ether. Insoluble in water.
Notes
Incompatible with strong oxidizing agents, strong bases and strong reducing agents.
RUO – Research Use Only
General References:
- Zhang, Z.; Xie, C.; Tan, X.; Song, G.; Wen, L.; Gao, H.; Ma, C. I2-catalyzed one-pot synthesis of pyrrolo[1,2-a]quinoxaline and imidazo[1,5-a]quinoxaline derivatives via sp3 and sp2 C-H cross-dehydrogenative coupling. Org. Chem. Front. 2015, 2 (8), 942-946.
- Vivekanand, T.; Sandhya, T.; Vinoth, P.; Nagarajan, S.; Maheswari, C. U.; Sridharan, V. N-(2-Aminobenzylidene)-4-methylanilines-stable and cheap alternate for 2-aminobenzaldehydes: concise synthesis of 3-unsubstituted-2-aroylindoles. Tetrahedron Lett. 2015, 56 (38), 5291-5294.