Search
Thermo Scientific Chemicals
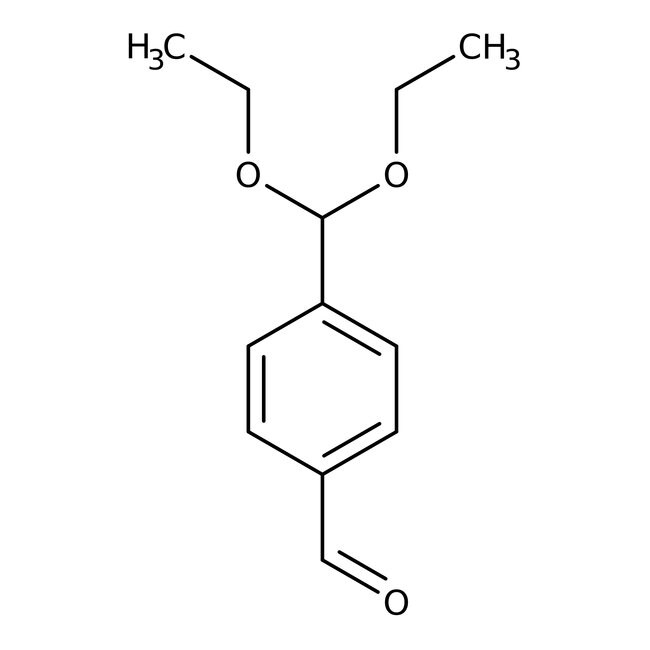
Terephthalaldehyde mono(diethyl acetal), 97%, stab.
CAS: 81172-89-6 | C12H16O3 | 208.26 g/mol
化学物質識別子
CAS81172-89-6
仕様 スペックシート
スペックシート
Appearance (Color)Clear colorless to yellow
Assay (GC)≥96.0%
Refractive Index1.5050-1.5090 @ 20?C
FormLiquid
Stabilizer0.1% triethylamine
4-(Diethoxymethyl)benzaldehyde was used in the synthesis of 4-(diethoxymethyl)benzyl alcohol.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-(Diethoxymethyl)benzaldehyde was used in the synthesis of 4-(diethoxymethyl)benzyl alcohol.
Solubility
soluble in alcohol, chlorinated solvents and toluene. Decomposes in water.
Notes
Air sensitive. Store under inert gas. Keep way from oxidizing agents, air.
4-(Diethoxymethyl)benzaldehyde was used in the synthesis of 4-(diethoxymethyl)benzyl alcohol.
Solubility
soluble in alcohol, chlorinated solvents and toluene. Decomposes in water.
Notes
Air sensitive. Store under inert gas. Keep way from oxidizing agents, air.
RUO – Research Use Only
General References:
- Yoshitsugu Akiyama; Yukio Nagasaki; Kazunori Kataoka. Synthesis of heterotelechelic poly(ethylene glycol) derivatives having alpha-benzaldehyde and μ-pyridyl disulfide groups by ring opening polymerization of ethylene oxide using 4-(diethoxymethyl)benzyl alkoxide as a novel initiator. Bioconjugate Chemistry. 2004, 15, (2), 424-427.
- Kyle J. Eash; Michael S. Pulia; Laura C. Wieland and Ram S. Mohan. A Simple Chemoselective Method for the Deprotection of Acetals and Ketals Using Bismuth Nitrate Pentahydrate. J. Org. Chem. 2000, 65(24), 8399-8401.