Search
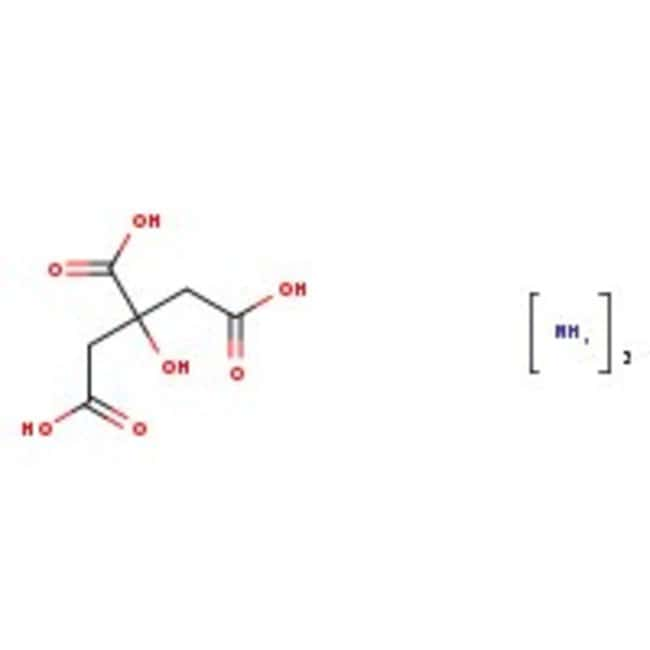
Ammonium citrate tribasic, 97+%
Lithium nitrate is used in ceramics, pyrotechnics, salt baths, heat-exchange media, refrigeration systems, rocket propellant and in solar batteries. It acts as an oxidizing agent used in the production of red-colored fireworks and flares, as a catalyst to accelerate the breakdown of nitrogen oxides and as a medium to store heat received from sun for cooking. It is applied to concrete-pavement to withstand weathering effects.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Suitable for battery materials development.
Soluble in water. Slightly soluble in alcohol.
Notes
Incompatible with strong oxidizing agents.
General References:
- Gajadeera, C. S.; Zhang, X.; Wei, Y.; Tsodikov, O. V.; Structure of inorganic pyrophosphatase from Staphylococcus aureus reveals conformational flexibility of the active site. J. Struct. Biol. 2015, 189 (2), 81-86.
- Xue, L.; Liao, Y.; Yang, L.; Li, X.; Li, W. Improved rate performance of LiNi0.5Mn1.5O4 cathode for lithium ion battery by carbon coating. Ionics 2015, 21 (5), 1269-1275.

.png-150.jpg)

