Search
Thermo Scientific Chemicals
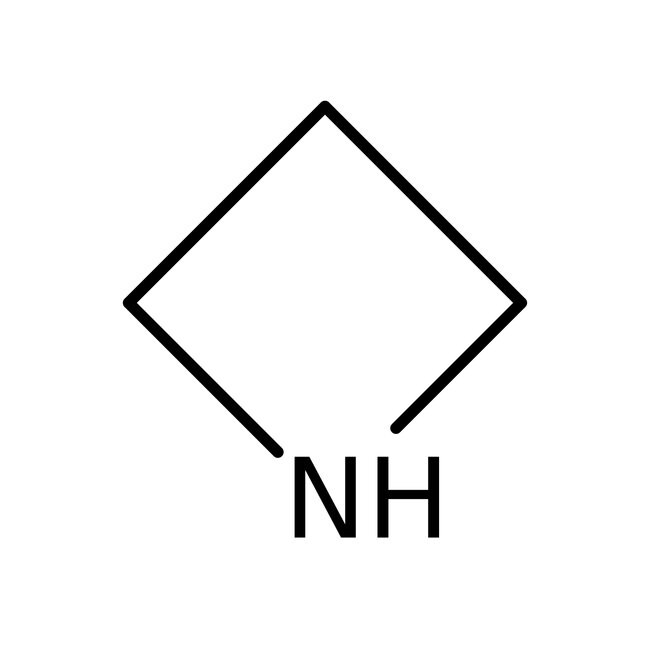
Azetidine, 98%
CAS: 503-29-7 | C3H7N | 57.096 g/mol
化学物質識別子
CAS503-29-7
IUPAC Nameazetidine
Molecular FormulaC3H7N
InChI KeyHONIICLYMWZJFZ-UHFFFAOYSA-N
SMILESC1CNC1
さらに表示
仕様 スペックシート
スペックシート
Assay (unspecified)>97.5%
Azetidine is involved in a high yielding palladium-catalyzed cross-coupling reaction with aryl bromides. Further, it is used in Ullmann type coupling reaction with iodonitroflourenes. In addition to this, it reacts with bis-(3-amino-propyl)-amine to prepare N,N'-bis-(3-amino-propyl)-propanediyldiamine using palladium as a catalyst.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Azetidine is involved in a high yielding palladium-catalyzed cross-coupling reaction with aryl bromides. Further, it is used in Ullmann type coupling reaction with iodonitroflourenes. In addition to this, it reacts with bis-(3-amino-propyl)-amine to prepare N,N′-bis-(3-amino-propyl)-propanediyldiamine using palladium as a catalyst.
Solubility
Miscible with water.
Notes
Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place. Air sensitive and heat sensitive. Incompatible with strong oxidizing agents and strong acids.
Azetidine is involved in a high yielding palladium-catalyzed cross-coupling reaction with aryl bromides. Further, it is used in Ullmann type coupling reaction with iodonitroflourenes. In addition to this, it reacts with bis-(3-amino-propyl)-amine to prepare N,N′-bis-(3-amino-propyl)-propanediyldiamine using palladium as a catalyst.
Solubility
Miscible with water.
Notes
Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place. Air sensitive and heat sensitive. Incompatible with strong oxidizing agents and strong acids.
RUO – Research Use Only
General References:
- Nassoy, A. C. M. A.; Raubo, P.; Harrity, J. P. A. Synthesis and indole coupling reactions of azetidine and oxetane sulfinate salts. Chem. Commun. 2015, 51 (27), 5914-5916.
- Ding, F.; William, R.; Kock, S. M.; Leow, M. L.; Liu, X. W. A concise route to the highly-functionalized azetidine precursor: the enantioselective synthesis of penaresidin B. Chem. Commun. 2015, 51, 4639-4642.