Search
Thermo Scientific Chemicals
PIPES, 0.5M buffer soln., pH 7.0
CAS: 5625-37-6 | C8H18N2O6S2 | 302.36 g/mol
仕様
CAS5625-37-6
ChEBICHEBI:44933
化学物質名または材質PIPES buffer soln.
色Colorless
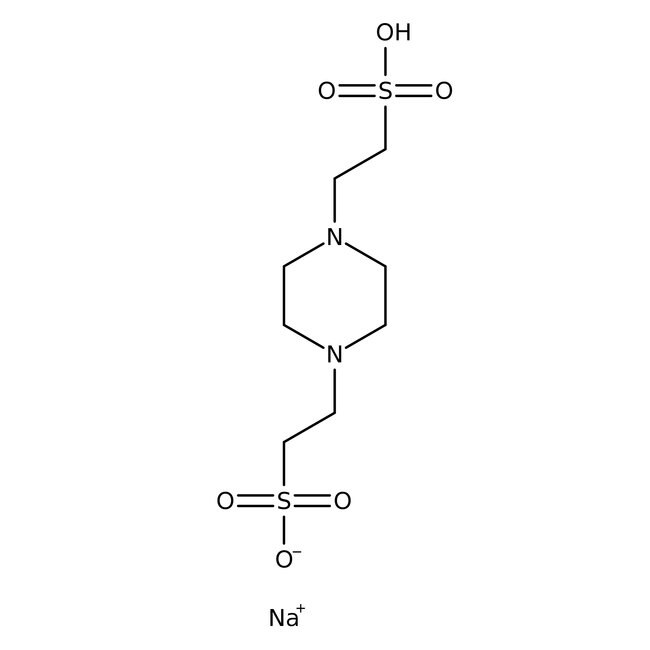
IUPAC名2-[4-(2-sulfoethyl)piperazin-1-yl]ethanesulfonic acid
さらに表示
PIPES, is used as a buffering agent in biochemistry. PIPES has pKa near the physiological pH which makes it useful in cell culture work. PIPES has been documented minimizing lipid loss when buffering glutaraldehyde histology in plant and animal tissues.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
PIPES, is used as a buffering agent in biochemistry. PIPES has pKa near the physiological pH which makes it useful in cell culture work. PIPES has been documented minimizing lipid loss when buffering glutaraldehyde histology in plant and animal tissues.
Solubility
Soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from strong oxidizing agents.
PIPES, is used as a buffering agent in biochemistry. PIPES has pKa near the physiological pH which makes it useful in cell culture work. PIPES has been documented minimizing lipid loss when buffering glutaraldehyde histology in plant and animal tissues.
Solubility
Soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from strong oxidizing agents.
RUO – Research Use Only
General References:
- MJ Alowitz.; MM Scherer. Kinetics of nitrate, nitrite, and Cr (VI) reduction by iron metal. Environmental Science & Technology.2002, 36, (3), 299-306.
- JB Olmsted, GG Borisy. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry. 1975, 14, (13), 2996-3005.