Search Thermo Fisher Scientific
Thermo Scientific Chemicals
1-Propylphosphonic acid cyclic anhydride, 50+% soln. in ethyl acetate, Thermo Scientific Chemicals
製品番号(カタログ番号): L11911.18
50 g, Each
化学物質識別子
CAS
68957-94-8
IUPAC Name
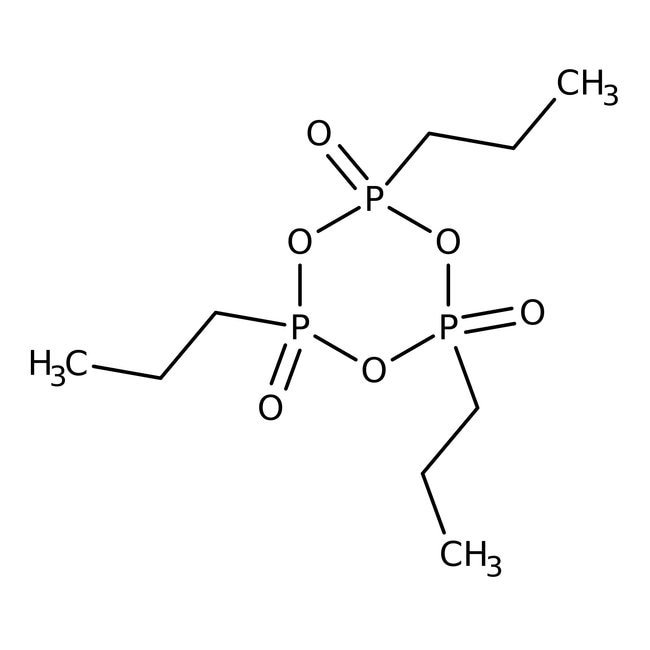
tripropyl-1,3,5,2λ⁵,4λ⁵,6λ⁵-trioxatriphosphinane-2,4,6-trione
Molecular Formula
C9H21O6P3
InChI Key
PAQZWJGSJMLPMG-UHFFFAOYSA-N
SMILES
CCCP1(=O)OP(=O)(CCC)OP(=O)(CCC)O1
仕様
Appearance (Color)
Clear colorless to yellow to brown
Assay from Suppliers CofA
≤50.0% w/w (Ethyl acetate - GC)
Phosphorus-31 NMR
≤0.5% w/w (Propanphosphonic acid)
Assay (Titration ex Anhydride)
≥50.0% w/w
Identification (FTIR)
Conforms
概要
1-Propylphosphonic acid cyclic anhydride is used in the synthesis of N-acyloxythiazole-2(3H)-thiones. It is also used to promote the synthesis of hydroxamic acids from carboxylic acids. Further, it is employed as an efficient promoter for the Lossen rearrangement to synthesis urea and carbamate derivatives.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Propylphosphonic acid cyclic anhydride is used in the synthesis of N-acyloxythiazole-2(3H)-thiones. It is also used to promote the synthesis of hydroxamic acids from carboxylic acids. Further, it is employed as an efficient promoter for the Lossen rearrangement to synthesis urea and carbamate derivatives.
Solubility
Miscible with dichloromethane, dimethyl formamide, dioxane, terahydrofuran, polar and aprotic organic solvents.
Notes
Moisture sensitive. Incompatible withstrong oxidizing agents.
1-Propylphosphonic acid cyclic anhydride is used in the synthesis of N-acyloxythiazole-2(3H)-thiones. It is also used to promote the synthesis of hydroxamic acids from carboxylic acids. Further, it is employed as an efficient promoter for the Lossen rearrangement to synthesis urea and carbamate derivatives.
Solubility
Miscible with dichloromethane, dimethyl formamide, dioxane, terahydrofuran, polar and aprotic organic solvents.
Notes
Moisture sensitive. Incompatible withstrong oxidizing agents.
RUO – Research Use Only
図
ドキュメントおよびダウンロード
証明書
ロット番号または部分ロット番号で検索
よくあるご質問(FAQ)
引用および参考文献
Search citations by name, author, journal title or abstract text