Search Thermo Fisher Scientific
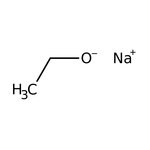
Sodium ethoxide, 21% w/w in ethanol, Thermo Scientific Chemicals
化学物質識別子
仕様
概要
Sodium ethoxide, 21% w/w in ethanol is used as a strong base in organic synthesis. It finds application in various chemical reactions such as condensation, esterification, alkoxylation and etherifcation. It is actively involved in Claisen condensation, Stobbe reaction and Wolf-kishner reduction. It is an important starting material for the synthesis of ethyl ester and diethyl ester of malonic acid. In Williamson ether synthesis, it reacts with ethyl bromide to form diethyl ether.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Sodium ethoxide, 21% w/w in ethanol is used as a strong base in organic synthesis. It finds application in various chemical reactions such as condensation, esterification, alkoxylation and etherifcation. It is actively involved in Claisen condensation, Stobbe reaction and Wolf-kishner reduction. It is an important starting material for the synthesis of ethyl ester and diethyl ester of malonic acid. In Williamson ether synthesis, it reacts with ethyl bromide to form diethyl ether.
Solubility
Miscible with alcohol.
Notes
Moisture sensitive. It hydrolyzes rapidly into sodium hydroxide in the presence of moist air. Incompatible with water, strong bases, acids, oxidizing agents, alkali metals, strong oxidizing agents, ammonia, acid chlorides, acid anhydrides, reducing agents, peroxides, acids and chlorinated solvents.