Search Thermo Fisher Scientific
Thermo Scientific Chemicals
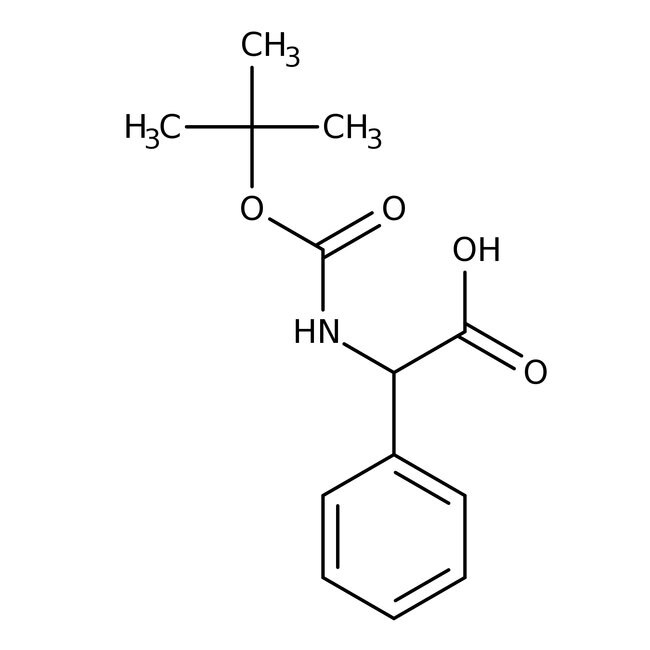
N-Boc-D-phenylglycine, 99%, Thermo Scientific Chemicals
製品番号(カタログ番号): L18540.03
1 g, Each
化学物質識別子
CAS
33125-05-2
IUPAC Name
2-{[(tert-butoxy)carbonyl]amino}-2-phenylacetic acid
Molecular Formula
C13H17NO4
InChI Key
HOBFSNNENNQQIU-UHFFFAOYNA-N
SMILES
CC(C)(C)OC(=O)NC(C(O)=O)C1=CC=CC=C1
仕様
Optical Rotation
-142.9 ± 0.1? (C=1 in ethanol)
Melting Point (clear melt)
88.0-95.0?C
Appearance (Color)
White
Assay (Aqueous acid-base Titration)
≥98.5 to ≤101.5%
Form
Powder
概要
It is a reagent of choice for assignment of absolute configuration of chiral primary amines by 1H NMR, giving better results than Mosher's acid ((R)-(+)-Methoxy-(trifluoromethyl)phenylacetic acid. αR)-α-[[(1,1-Dimethylethoxy)carbonyl]amino]-benzeneacetic Acid is D-(+)-2-Phenylglycine with Boc protecting group. (αR)-α-[[(1,1-Dimethylethoxy)carbonyl]amino]-benzeneacetic Acid is used as part of a catalyst combination to catalyze regioselective [4 + 2] cycloadditions of β-substituted cyclic enones and polyconjugated malononitriles. It can also be used to catalyze stereoselective preparation of polyfunctional nitrocyclohexene carboxaldehydes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is a reagent of choice for assignment of absolute configuration of chiral primary amines by 1H NMR, giving better results than Mosher′s acid ((R)-(+)-ɑ-Methoxy-ɑ-(trifluoromethyl)phenylacetic acid. αR)-α-[[(1,1-Dimethylethoxy)carbonyl]amino]-benzeneacetic Acid is D-(+)-2-Phenylglycine with Boc protecting group. (αR)-α-[[(1,1-Dimethylethoxy)carbonyl]amino]-benzeneacetic Acid is used as part of a catalyst combination to catalyze regioselective [4 + 2] cycloadditions of β-substituted cyclic enones and polyconjugated malononitriles. It can also be used to catalyze stereoselective preparation of polyfunctional nitrocyclohexene carboxaldehydes.
Solubility
Insoluble in water. Slightly soluble in DMSO and methanol.
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
It is a reagent of choice for assignment of absolute configuration of chiral primary amines by 1H NMR, giving better results than Mosher′s acid ((R)-(+)-ɑ-Methoxy-ɑ-(trifluoromethyl)phenylacetic acid. αR)-α-[[(1,1-Dimethylethoxy)carbonyl]amino]-benzeneacetic Acid is D-(+)-2-Phenylglycine with Boc protecting group. (αR)-α-[[(1,1-Dimethylethoxy)carbonyl]amino]-benzeneacetic Acid is used as part of a catalyst combination to catalyze regioselective [4 + 2] cycloadditions of β-substituted cyclic enones and polyconjugated malononitriles. It can also be used to catalyze stereoselective preparation of polyfunctional nitrocyclohexene carboxaldehydes.
Solubility
Insoluble in water. Slightly soluble in DMSO and methanol.
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
図
ドキュメントおよびダウンロード
証明書
ロット番号または部分ロット番号で検索
よくあるご質問(FAQ)
引用および参考文献
Search citations by name, author, journal title or abstract text