Search Thermo Fisher Scientific
Thermo Scientific Chemicals
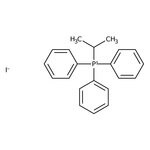
Isopropyltriphenylphosphonium iodide, 98+%, Thermo Scientific Chemicals
Catalog number: A12881.09
10 g, Each


Thermo Scientific Chemicals
Isopropyltriphenylphosphonium iodide, 98+%, Thermo Scientific Chemicals
Catalog number: A12881.09
10 g, Each
Quantity
Catalog number: A12881.09
also known as A12881-09
Price (USD)
Price: 42.50
Online price: 37.65
Your price:
Quantity
-
Chemical Identifiers
CAS
24470-78-8
IUPAC Name
triphenyl(propan-2-yl)phosphanium iodide
Molecular Formula
C21H22IP
InChI Key
HHBXWXJLQYJJBW-UHFFFAOYSA-M
SMILES
[I-].CC(C)[P+](C1=CC=CC=C1)(C1=CC=CC=C1)C1=CC=CC=C1
Specifications
Elemental Analysis
I: 28.48-30.24% (theory 29.36%)
Appearance (Color)
White to pale cream or pale yellow
Form
Crystalline powder or powder
Elemental Analysis
C: 56.60-60.10% (theory 58.35%)
Water Content (Karl Fischer Titration)
≤1.0%
Description
Useful precursor of isopropylidene unit by Wittig reaction with aldehydes. It is also used in tandem cyclopropanation and Wittig olefination in a synthesis of chrysanthemic acid. Also employed in Wittig and cyclopropanation reactions. It is used as a reactant for total synthesis of heliananes, in the preparation of highly substituted benzene derivatives, synthesis of curcuphenol and elvirol analogs via a retro-aldol reaction as fungicidal agents, 4-substituted methoxylbenzoyl-aryl-thiazoles analogues as potent and orally bioavailable anticancer agents.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Useful precursor of isopropylidene unit by Wittig reaction with aldehydes. It is also used in tandem cyclopropanation and Wittig olefination in a synthesis of chrysanthemic acid. Also employed in Wittig and cyclopropanation reactions. It is used as a reactant for total synthesis of heliananes, in the preparation of highly substituted benzene derivatives, synthesis of curcuphenol and elvirol analogs via a retro-aldol reaction as fungicidal agents, 4-substituted methoxylbenzoyl-aryl-thiazoles analogues as potent and orally bioavailable anticancer agents.
Solubility
Solubility in methanol, very faint turbidity.
Notes
Light sensitive and hygroscopic. Store away from oxidizing agents and light. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
Useful precursor of isopropylidene unit by Wittig reaction with aldehydes. It is also used in tandem cyclopropanation and Wittig olefination in a synthesis of chrysanthemic acid. Also employed in Wittig and cyclopropanation reactions. It is used as a reactant for total synthesis of heliananes, in the preparation of highly substituted benzene derivatives, synthesis of curcuphenol and elvirol analogs via a retro-aldol reaction as fungicidal agents, 4-substituted methoxylbenzoyl-aryl-thiazoles analogues as potent and orally bioavailable anticancer agents.
Solubility
Solubility in methanol, very faint turbidity.
Notes
Light sensitive and hygroscopic. Store away from oxidizing agents and light. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text