Search Thermo Fisher Scientific
Thermo Scientific Chemicals
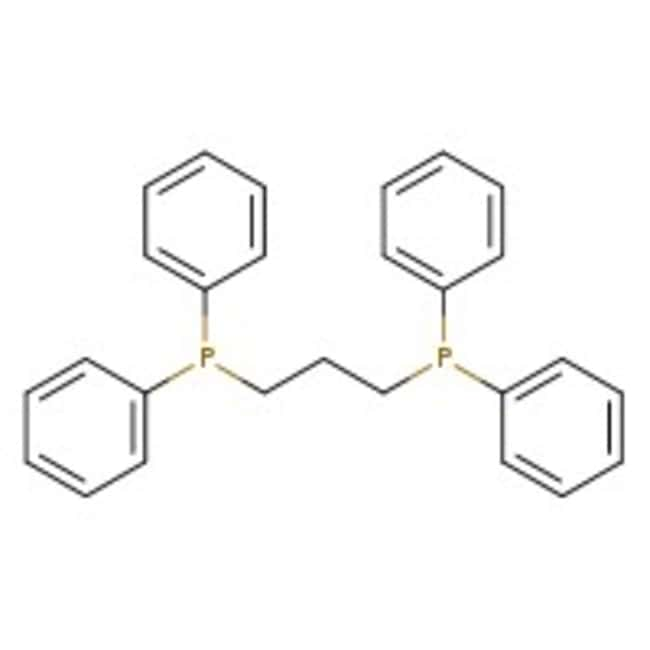
1,3-Bis(diphenylphosphino)propane, 97%, Thermo Scientific Chemicals
Catalog number: A12931.14
25 g, Each

Thermo Scientific Chemicals
1,3-Bis(diphenylphosphino)propane, 97%, Thermo Scientific Chemicals
Catalog number: A12931.14
25 g, Each
Quantity
Catalog number: A12931.14
also known as A12931-14
Price (USD)
Price: 248.00
Online price: 218.65
Your price:
Quantity
-
Chemical Identifiers
CAS
6737-42-4
IUPAC Name
[3-(diphenylphosphanyl)propyl]diphenylphosphane
Molecular Formula
C27H26P2
InChI Key
LVEYOSJUKRVCCF-UHFFFAOYSA-N
SMILES
C(CP(C1=CC=CC=C1)C1=CC=CC=C1)CP(C1=CC=CC=C1)C1=CC=CC=C1
Specifications
Form
Powder
Appearance (Color)
White to very pale yellow
Purity (DSC)
>96.0%
Phosphorus-31 NMR
Conforms to structure
Description
1,3-Bis(diphenylphosphino)propane is used as a bidentate ligand in coordination chemistry and form complex dichloro(1,3-bis(diphenylphosphino)propane)nickel by reacting with nickel(II) chloride. This complex is used as a catalyst for Kumada coupling reaction. It also acts as a ligand for palladium(II) catalysts which is useful for the co-polymerization of carbon monoxide and ethylene to get polyketones. Further, it is employed in palladium-catalyzed arylation under Heck reaction and Suzuki reaction. In addition to this, it serves as a catalyst for Negishi coupling and Sonogashira coupling reactions.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,3-Bis(diphenylphosphino)propane is used as a bidentate ligand in coordination chemistry and form complex dichloro(1,3-bis(diphenylphosphino)propane)nickel by reacting with nickel(II) chloride. This complex is used as a catalyst for Kumada coupling reaction. It also acts as a ligand for palladium(II) catalysts which is useful for the co-polymerization of carbon monoxide and ethylene to get polyketones. Further, it is employed in palladium-catalyzed arylation under Heck reaction and Suzuki reaction. In addition to this, it serves as a catalyst for Negishi coupling and Sonogashira coupling reactions.
Solubility
Insoluble in water.
Notes
Air sensitive. Incompatible with strong oxidizing agents.
1,3-Bis(diphenylphosphino)propane is used as a bidentate ligand in coordination chemistry and form complex dichloro(1,3-bis(diphenylphosphino)propane)nickel by reacting with nickel(II) chloride. This complex is used as a catalyst for Kumada coupling reaction. It also acts as a ligand for palladium(II) catalysts which is useful for the co-polymerization of carbon monoxide and ethylene to get polyketones. Further, it is employed in palladium-catalyzed arylation under Heck reaction and Suzuki reaction. In addition to this, it serves as a catalyst for Negishi coupling and Sonogashira coupling reactions.
Solubility
Insoluble in water.
Notes
Air sensitive. Incompatible with strong oxidizing agents.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text