Search Thermo Fisher Scientific
Thermo Scientific Chemicals
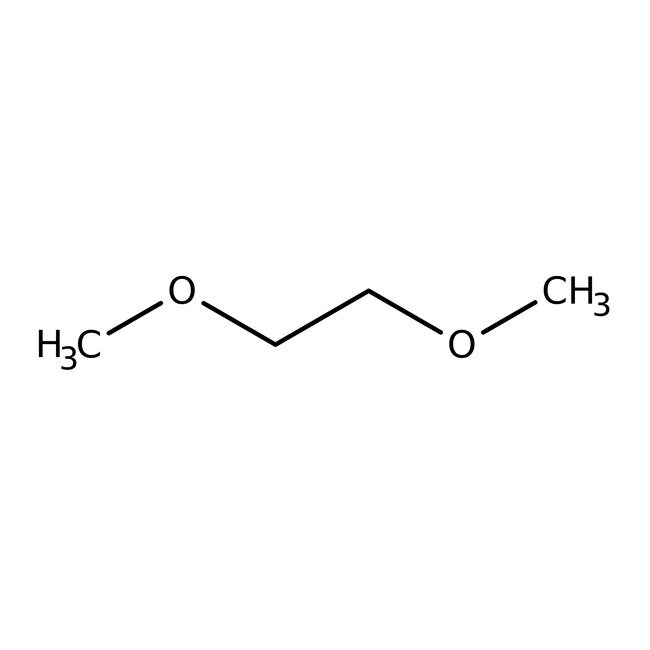
1,2-Dimethoxyethane, 99+%, stab. with BHT, Thermo Scientific Chemicals
Catalog number: A12986.AE
100 mL, Each


Thermo Scientific Chemicals
1,2-Dimethoxyethane, 99+%, stab. with BHT, Thermo Scientific Chemicals
Catalog number: A12986.AE
100 mL, Each
Quantity
Chemical Identifiers
CAS
110-71-4
IUPAC Name
1,2-dimethoxyethane
Molecular Formula
C4H10O2
InChI Key
XTHFKEDIFFGKHM-UHFFFAOYSA-N
SMILES
COCCOC
Specifications
Water Content (Karl Fischer Titration)
≤0.1%
Appearance (Color)
Clear colorless
Form
Liquid
Assay (GC)
≥99.0%
Identification (FTIR)
Conforms
Description
1,2-Dimethoxyethane is widely used as a solvent for electrolyte of lithium batteries, polysilicones, oligo- and polysaccharides. It plays an important role in Grignard reactions, Suzuki reactions and Stille couplings in organometallic chemistry and in pharmaceutical synthesis. It is a higher boiling point solvent and is used as an alternative to diethyl ether and tetrahydrofuran. It is used for the etching of synthetic polymers like polytetrafluoroethylene and other fluoropolymers with alkali metal dispersions.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,2-Dimethoxyethane is widely used as a solvent for electrolyte of lithium batteries, polysilicones, oligo- and polysaccharides. It plays an important role in Grignard reactions, Suzuki reactions and Stille couplings in organometallic chemistry and in pharmaceutical synthesis. It is a higher boiling point solvent and is used as an alternative to diethyl ether and tetrahydrofuran. It is used for the etching of synthetic polymers like polytetrafluoroethylene and other fluoropolymers with alkali metal dispersions.
Solubility
Miscible with water, methanol, ethanol, diethyl ether, acetone, tetrahydrofuran, benzene and toluene.
Notes
Keep away from sources of ignition. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents. It can be oxidized on air to form peroxides.
1,2-Dimethoxyethane is widely used as a solvent for electrolyte of lithium batteries, polysilicones, oligo- and polysaccharides. It plays an important role in Grignard reactions, Suzuki reactions and Stille couplings in organometallic chemistry and in pharmaceutical synthesis. It is a higher boiling point solvent and is used as an alternative to diethyl ether and tetrahydrofuran. It is used for the etching of synthetic polymers like polytetrafluoroethylene and other fluoropolymers with alkali metal dispersions.
Solubility
Miscible with water, methanol, ethanol, diethyl ether, acetone, tetrahydrofuran, benzene and toluene.
Notes
Keep away from sources of ignition. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents. It can be oxidized on air to form peroxides.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text