Search Thermo Fisher Scientific
Thermo Scientific Chemicals
2-Hydroxyisobutyric acid, 99% (dry wt.), water <2%, Thermo Scientific Chemicals
Catalog number: A13146.22
100 g, Each


Thermo Scientific Chemicals
2-Hydroxyisobutyric acid, 99% (dry wt.), water <2%, Thermo Scientific Chemicals
Catalog number: A13146.22
100 g, Each
Quantity
Catalog number: A13146.22
also known as A13146-22
Price (USD)
Special offer:
79.65 93.70 Save 14.05 (14%)
Ends: 15-Oct-2024
Each
Quantity
-
Chemical Identifiers
CAS
594-61-6
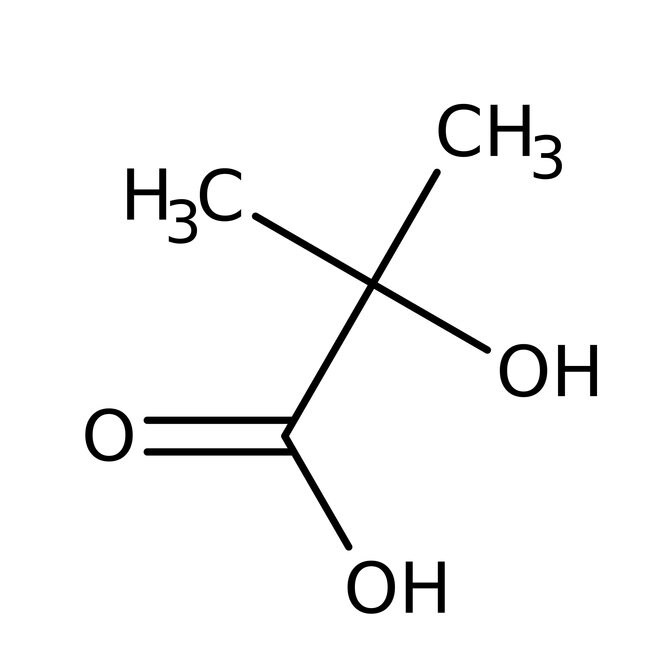
IUPAC Name
2-hydroxy-2-methylpropanoic acid
Molecular Formula
C4H8O3
InChI Key
BWLBGMIXKSTLSX-UHFFFAOYSA-N
SMILES
CC(C)(O)C(O)=O
Specifications
Appearance (Color)
White to pale cream
Assay (Aqueous acid-base Titration)
≥98.5 to ≤101.5% (dry wt. basis)
Assay (Silylated GC)
≥98.5%
Water Content (Karl Fischer Titration)
≤2.0%
Melting Point (clear melt)
75-81?C
Description
2-Hydroxyisobutyric acid is used as an electrolyte in the separation of the metal ions by on-line cyclic voltammetry. It is also used as a building block for the synthesis of polymers. Its conjugate base, 2-hydroxybutyrate is utilized to catabolize L-threonine as well as synthesize glutathione. Further, it is used as an indicator for the early detection insulin resistance in non-diabetic subjects.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Hydroxyisobutyric acid is used as an electrolyte in the separation of the metal ions by on-line cyclic voltammetry. It is also used as a building block for the synthesis of polymers. Its conjugate base, 2-hydroxybutyrate is utilized to catabolize L-threonine as well as synthesize glutathione. Further, it is used as an indicator for the early detection insulin resistance in non-diabetic subjects.
Solubility
Soluble in water, ether, alcohol, methanol and hot benzene.
Notes
Incompatible with oxidizing agents, bases and reducing agents.
2-Hydroxyisobutyric acid is used as an electrolyte in the separation of the metal ions by on-line cyclic voltammetry. It is also used as a building block for the synthesis of polymers. Its conjugate base, 2-hydroxybutyrate is utilized to catabolize L-threonine as well as synthesize glutathione. Further, it is used as an indicator for the early detection insulin resistance in non-diabetic subjects.
Solubility
Soluble in water, ether, alcohol, methanol and hot benzene.
Notes
Incompatible with oxidizing agents, bases and reducing agents.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text