Search Thermo Fisher Scientific
Thermo Scientific Chemicals
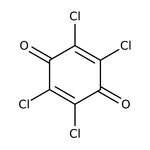
p-Chloranil, 97%, Thermo Scientific Chemicals
Catalog number: A13495.22
100 g, Each


Thermo Scientific Chemicals
p-Chloranil, 97%, Thermo Scientific Chemicals
Catalog number: A13495.22
100 g, Each
Quantity
Catalog number: A13495.22
also known as A13495-22
Price (USD)
Price: 119.00
Online price: 101.65
Your price:
Quantity
-
Chemical Identifiers
CAS
118-75-2
Specifications
Form
Crystals or powder or crystalline powder
Identification (FTIR)
Conforms
Appearance (Color)
Yellow to yellow-green
Assay (HPLC)
≥96.0%
Description
p-Chloranil is used as a dye intermediate, oxidizing agent, vulcanizing agent and dehydrogenation reagent. It is also used to make chloranil electrodes for pH measurements. Further, it serves as a hydrogen acceptor and used for the aromatization reactions such as conversion of cyclohexadienes to the benzene derivatives. It is used to test the secondary amine. In addition to this, it is employed as a precursor to prepare diaziquone, which is used as a cancer chemotherapeutic agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
p-Chloranil is used as a dye intermediate, oxidizing agent, vulcanizing agent and dehydrogenation reagent. It is also used to make chloranil electrodes for pH measurements. Further, it serves as a hydrogen acceptor and used for the aromatization reactions such as conversion of cyclohexadienes to the benzene derivatives. It is used to test the secondary amine. In addition to this, it is employed as a precursor to prepare diaziquone, which is used as a cancer chemotherapeutic agent.
Solubility
Soluble in ether and acetone. Slightly soluble in chloroform, dimethylformamide, solvent naphtha, benzene, carbon disulfide and carbon tetrachloride. Insoluble in water, cold petroleum ether, methanol, dibutyl phthalate and cold alcohol.
Notes
Incompatible with strong oxidizing agents and strong bases.
p-Chloranil is used as a dye intermediate, oxidizing agent, vulcanizing agent and dehydrogenation reagent. It is also used to make chloranil electrodes for pH measurements. Further, it serves as a hydrogen acceptor and used for the aromatization reactions such as conversion of cyclohexadienes to the benzene derivatives. It is used to test the secondary amine. In addition to this, it is employed as a precursor to prepare diaziquone, which is used as a cancer chemotherapeutic agent.
Solubility
Soluble in ether and acetone. Slightly soluble in chloroform, dimethylformamide, solvent naphtha, benzene, carbon disulfide and carbon tetrachloride. Insoluble in water, cold petroleum ether, methanol, dibutyl phthalate and cold alcohol.
Notes
Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text