Search Thermo Fisher Scientific
Thermo Scientific Chemicals
N-Hydroxyphthalimide, 98+%, Thermo Scientific Chemicals
Catalog number: A13862.22
100 g, Each


Thermo Scientific Chemicals
N-Hydroxyphthalimide, 98+%, Thermo Scientific Chemicals
Catalog number: A13862.22
100 g, Each
Quantity
Catalog number: A13862.22
Price (USD)
Price: 43.70
Online price: 37.65
Your price:
Quantity
-
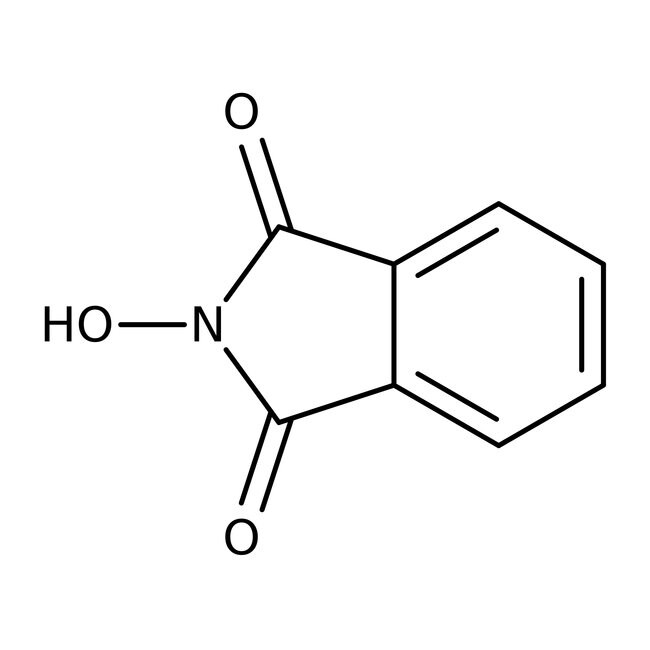
Chemical Identifiers
CAS
524-38-9
IUPAC Name
2-hydroxy-2,3-dihydro-1H-isoindole-1,3-dione
Molecular Formula
C8H5NO3
InChI Key
CFMZSMGAMPBRBE-UHFFFAOYSA-N
SMILES
ON1C(=O)C2=CC=CC=C2C1=O
Specifications
Appearance (Color)
White to yellow
Identification (FTIR)
Conforms
Assay (Aqueous acid-base Titration)
≥97.5 to ≤102.5%
Form
Powder
Description
Aerobic oxidation of various alcohols has been accomplished by using a new catalytic system, N-hydroxyphthalimide (NHPI) combined with Co (acac). A practical catalytic method to convert alkylbenzenes into the corresponding carboxylic acids under atmospheric dioxygen at ambient temperature using a combined catalytic system consisting of N-hydroxyphthalimide (NHPI) and Co (OAc) 2 was developed. Novel catalysis by N-hydroxyphthalimide in the oxidation of organic substrates by molecular oxygen was described. Free radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide. Purely organic and catalytic systems of anthraquinones and N-hydroxyphthalimide efficiently promote oxygenation of hydrocarbons with dioxygen under mild conditions. Hydroxylation of polycyclic alkanes with molecular oxygen catalyzed by N-hydroxyphthalimide (NHPI) combined with was transition metal salts.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Aerobic oxidation of various alcohols has been accomplished by using a new catalytic system, N-hydroxyphthalimide (NHPI) combined with Co (acac). A practical catalytic method to convert alkylbenzenes into the corresponding carboxylic acids under atmospheric dioxygen at ambient temperature using a combined catalytic system consisting of N-hydroxyphthalimide (NHPI) and Co (OAc) 2 was developed. Novel catalysis by N-hydroxyphthalimide in the oxidation of organic substrates by molecular oxygen was described. Free radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide. Purely organic and catalytic systems of anthraquinones and N-hydroxyphthalimide efficiently promote oxygenation of hydrocarbons with dioxygen under mild conditions. Hydroxylation of polycyclic alkanes with molecular oxygen catalyzed by N-hydroxyphthalimide (NHPI) combined with was transition metal salts.
Solubility
Slightly soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
Aerobic oxidation of various alcohols has been accomplished by using a new catalytic system, N-hydroxyphthalimide (NHPI) combined with Co (acac). A practical catalytic method to convert alkylbenzenes into the corresponding carboxylic acids under atmospheric dioxygen at ambient temperature using a combined catalytic system consisting of N-hydroxyphthalimide (NHPI) and Co (OAc) 2 was developed. Novel catalysis by N-hydroxyphthalimide in the oxidation of organic substrates by molecular oxygen was described. Free radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide. Purely organic and catalytic systems of anthraquinones and N-hydroxyphthalimide efficiently promote oxygenation of hydrocarbons with dioxygen under mild conditions. Hydroxylation of polycyclic alkanes with molecular oxygen catalyzed by N-hydroxyphthalimide (NHPI) combined with was transition metal salts.
Solubility
Slightly soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text