Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Sodium iodide, 99+% (dry wt., water <1.5%), Thermo Scientific Chemicals
Catalog number: A15480.22
100 g, Each

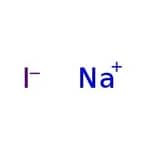

Thermo Scientific Chemicals
Sodium iodide, 99+% (dry wt., water <1.5%), Thermo Scientific Chemicals
Catalog number: A15480.22
100 g, Each
Quantity
Catalog number: A15480.22
also known as A15480-22
Price (USD)
Price: 54.50
Online price: 46.65
Your price:
Quantity
-
Chemical Identifiers
CAS
7681-82-5
IUPAC Name
sodium iodide
Molecular Formula
INa
InChI Key
FVAUCKIRQBBSSJ-UHFFFAOYSA-M
SMILES
[Na+].[I-]
Specifications
Appearance (Color)
White
Loss on Drying
≤1.5% (200°C/constant weight)
Form
Crystals or powder or crystalline powder or fused solid
Assay (Titration ex Iodide)
≥99.0 to ≤101.0% (dry wt. basis)
Description
Sodium iodide is widely used for halide exchange (Finkelstein reaction), for example in the conversion of an alkyl chloride, allyl chloride and arylmethyl chloride into their respective iodides, which are precursors for pharmaceutical and fine chemical products. They are used to ehnance the efficiency of the formation of Wittig adducts from less reactive chlorides and bromides. Appropriate prepartions find use as a nutrient supplement. Sodium iodide is used as the precursor to the control agent in ab initio emulsion polymerization. Sodium iodide finds use in the determination of dissolved oxygen in the modified Winkler method, synthesis of the fluorescent dye coppersensor-1 (CS1) for imaging labile copper pools in biological samples, and the cleavage of esters, lactones, carbamates and ethers in combination with chlorotrimethylsilane.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Sodium iodide is widely used for halide exchange (Finkelstein reaction), for example in the conversion of an alkyl chloride, allyl chloride and arylmethyl chloride into their respective iodides, which are precursors for pharmaceutical and fine chemical products. They are used to ehnance the efficiency of the formation of Wittig adducts from less reactive chlorides and bromides. Appropriate prepartions find use as a nutrient supplement. Sodium iodide is used as the precursor to the control agent in ab initio emulsion polymerization. Sodium iodide finds use in the determination of dissolved oxygen in the modified Winkler method, synthesis of the fluorescent dye coppersensor-1 (CS1) for imaging labile copper pools in biological samples, and the cleavage of esters, lactones, carbamates and ethers in combination with chlorotrimethylsilane.
Solubility
Very soluble in water and liquid ammonia. Freely soluble in acetone, formic acid, formamide, methanol, and acetonitrile. Soluble in N,N-dimethylformamide.
Notes
Deliquescent. Store in a tightly closed container. Incompatible with strong oxidizing agents and acids.
Sodium iodide is widely used for halide exchange (Finkelstein reaction), for example in the conversion of an alkyl chloride, allyl chloride and arylmethyl chloride into their respective iodides, which are precursors for pharmaceutical and fine chemical products. They are used to ehnance the efficiency of the formation of Wittig adducts from less reactive chlorides and bromides. Appropriate prepartions find use as a nutrient supplement. Sodium iodide is used as the precursor to the control agent in ab initio emulsion polymerization. Sodium iodide finds use in the determination of dissolved oxygen in the modified Winkler method, synthesis of the fluorescent dye coppersensor-1 (CS1) for imaging labile copper pools in biological samples, and the cleavage of esters, lactones, carbamates and ethers in combination with chlorotrimethylsilane.
Solubility
Very soluble in water and liquid ammonia. Freely soluble in acetone, formic acid, formamide, methanol, and acetonitrile. Soluble in N,N-dimethylformamide.
Notes
Deliquescent. Store in a tightly closed container. Incompatible with strong oxidizing agents and acids.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text