Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Lead(IV) acetate, 96% (dry wt.), stab. with 5-10% glacial acetic acid, Thermo Scientific Chemicals
Catalog number: A15551.0B
1000 g, Each


Thermo Scientific Chemicals
Lead(IV) acetate, 96% (dry wt.), stab. with 5-10% glacial acetic acid, Thermo Scientific Chemicals
Catalog number: A15551.0B
1000 g, Each
Quantity
Chemical Identifiers
CAS
546-67-8
IUPAC Name
λ²-lead(2+) tetraacetate
Molecular Formula
C8H12O8Pb
InChI Key
ACKFDYCQCBEDNU-UHFFFAOYSA-J
SMILES
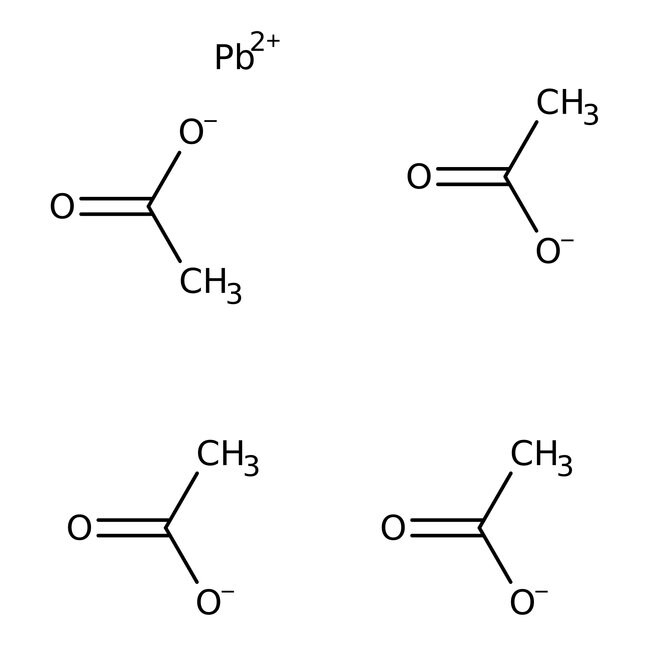
[Pb++].CC([O-])=O.CC([O-])=O.CC([O-])=O.CC([O-])=O
Specifications
Comment
May contain fine dark particles from the manufacturing process
Form
Moist crystals or powder or crystalline powder
Appearance (Color)
White to pale cream or pale grey
Assay from Supplier's CofA
≥95.0% (ex Pb; dry wt. basis, Iodometry)
Description
Lead(IV) acetate is an important oxidizing agent and a source of acetyloxy group used in organic synthesis. For example, 1,4-dioxene is prepared from dioxane involving 2-acetoxy-1,4-dioxane as an intermediate. Similarly, it is used for the preparation of bis(trifluoromethyl)diazomethane from hexafluoroacetone hydrazone. It also reacts with alkenes, alcohols having a delta-proton and di-n-butyl d-tartrate to get gamma-lactones, cyclic ethers and n-butyl glyoxylate respectively. It induces the cleavage of 1,2-diols to the corresponding aldehydes or ketones. It is actively involved in the Kochi reaction for the decarboxylation of carboxylic acids to alkyl halides and used as an alternative reagent to bromine in the Hofmann rearrangement.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Lead(IV) acetate is an important oxidizing agent and a source of acetyloxy group used in organic synthesis. For example, 1,4-dioxene is prepared from dioxane involving 2-acetoxy-1,4-dioxane as an intermediate. Similarly, it is used for the preparation of bis(trifluoromethyl)diazomethane from hexafluoroacetone hydrazone. It also reacts with alkenes, alcohols having a delta-proton and di-n-butyl d-tartrate to get gamma-lactones, cyclic ethers and n-butyl glyoxylate respectively. It induces the cleavage of 1,2-diols to the corresponding aldehydes or ketones. It is actively involved in the Kochi reaction for the decarboxylation of carboxylic acids to alkyl halides and used as an alternative reagent to bromine in the Hofmann rearrangement.
Solubility
Soluble in water, ethanol, chloroform, benzene, nitrobenzene, tetrachloroethane, nitric acid, hot acetic acid and hydrochloric acid.
Notes
Air and moisture sensitive. Store in cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with alcohols, strong acids and strong reducing agents.
Lead(IV) acetate is an important oxidizing agent and a source of acetyloxy group used in organic synthesis. For example, 1,4-dioxene is prepared from dioxane involving 2-acetoxy-1,4-dioxane as an intermediate. Similarly, it is used for the preparation of bis(trifluoromethyl)diazomethane from hexafluoroacetone hydrazone. It also reacts with alkenes, alcohols having a delta-proton and di-n-butyl d-tartrate to get gamma-lactones, cyclic ethers and n-butyl glyoxylate respectively. It induces the cleavage of 1,2-diols to the corresponding aldehydes or ketones. It is actively involved in the Kochi reaction for the decarboxylation of carboxylic acids to alkyl halides and used as an alternative reagent to bromine in the Hofmann rearrangement.
Solubility
Soluble in water, ethanol, chloroform, benzene, nitrobenzene, tetrachloroethane, nitric acid, hot acetic acid and hydrochloric acid.
Notes
Air and moisture sensitive. Store in cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with alcohols, strong acids and strong reducing agents.
WARNING: Cancer – www.P65Warnings.ca.gov
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text