Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Pyridine hydrobromide perbromide, tech. 90%, Thermo Scientific Chemicals
Catalog number: A15684.22
100 g, Each

Thermo Scientific Chemicals
Pyridine hydrobromide perbromide, tech. 90%, Thermo Scientific Chemicals
Catalog number: A15684.22
100 g, Each
Quantity
Catalog number: A15684.22
also known as A15684-22
Price (USD)
Price: 106.00
Online price: 90.65
Your price:
Quantity
-
Chemical Identifiers
CAS
39416-48-3
Molecular Formula
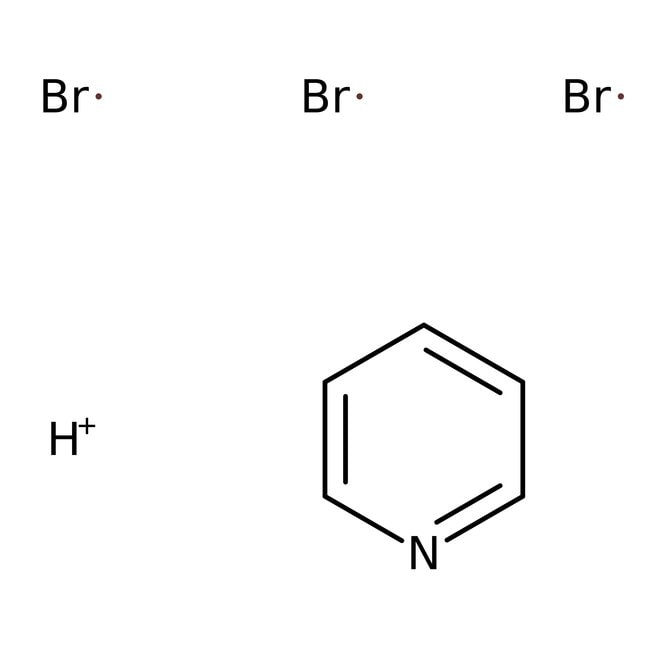
C5H6Br3N
InChI Key
GWJBDYRGIFGTOG-UHFFFAOYSA-O
SMILES
[H+].[Br].[Br].[Br].C1=CC=NC=C1
Molecular Weight (g/mol)
319.82
Specifications
Melting Point (clear melt)
129.5-136.5°C (non-U.S specification)
Form
Crystals or powder or crystalline powder or fused/lumpy solid
Assay from Supplier's CofA
≥88.0% (U.S. specification)
Appearance (Color)
Yellow to orange to red to red-brown
Assay (Titration ex Bromide)
≥88.0% (non-U.S. specification)
Description
Pyridine hydrobromide perbromide is used as a brominating reagent in alfa-bromination and alfa-thiocyanation of ketones, phenols, unsaturated and aromatic ethers. It is used as a raw material in the preparation of beta-adrenergic blocking agents. Furthermore, it is used as an analytical reagent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Pyridine hydrobromide perbromide is used as a brominating reagent in alfa-bromination and alfa-thiocyanation of ketones, phenols, unsaturated and aromatic ethers. It is used as a raw material in the preparation of beta-adrenergic blocking agents. Furthermore, it is used as an analytical reagent.
Solubility
Soluble in methanol, acetic acid, ethanol, n-butanol and terahydrofuran. Insoluble in water, carbon tetrachloride, ethyl bromide, benzene, toluene, ligroin, petroleum ether.
Notes
Store in cool place. Incompatible with strong oxidizing agents and strong bases.
Pyridine hydrobromide perbromide is used as a brominating reagent in alfa-bromination and alfa-thiocyanation of ketones, phenols, unsaturated and aromatic ethers. It is used as a raw material in the preparation of beta-adrenergic blocking agents. Furthermore, it is used as an analytical reagent.
Solubility
Soluble in methanol, acetic acid, ethanol, n-butanol and terahydrofuran. Insoluble in water, carbon tetrachloride, ethyl bromide, benzene, toluene, ligroin, petroleum ether.
Notes
Store in cool place. Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text