Search Thermo Fisher Scientific
Propargyl bromide, 80% in toluene, stab. with MgO, Thermo Scientific Chemicals
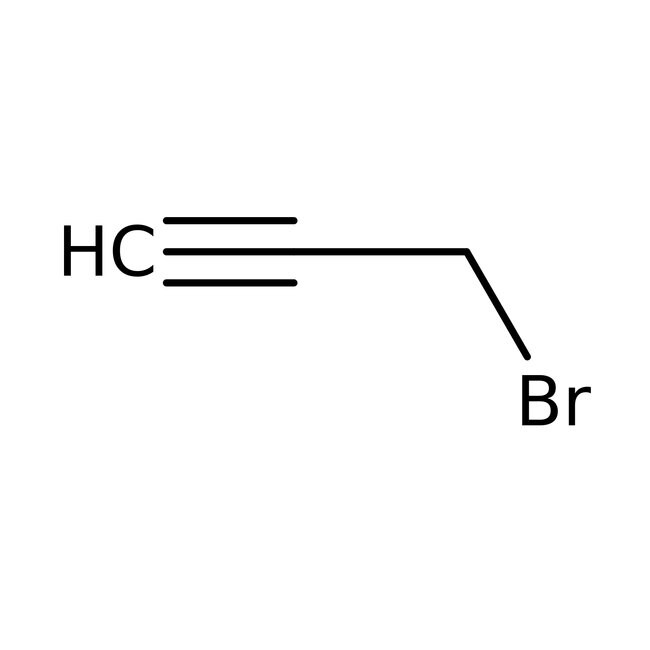


Propargyl bromide, 80% in toluene, stab. with MgO, Thermo Scientific Chemicals
Chemical Identifiers
Specifications
Description
Propargyl bromide, 80% in toluene is used to prepare betulonic acid-peptide conjugates with anti-inflammatory activity and propargylic amines employed in enyne metathesis. It finds application in the propargylation of spiro ketones, allylic alcohols and enone complexes. In Barbier-type reaction, it reacts with aldehydes to give alkyne alcohols. It is actively involved in the synthesis of polyethylene glycol (PEG) and peptide-grafted polyesters.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Propargyl bromide, 80% in toluene is used to prepare betulonic acid-peptide conjugates with anti-inflammatory activity and propargylic amines employed in enyne metathesis. It finds application in the propargylation of spiro ketones, allylic alcohols and enone complexes. In Barbier-type reaction, it reacts with aldehydes to give alkyne alcohols. It is actively involved in the synthesis of polyethylene glycol (PEG) and peptide-grafted polyesters.
Solubility
Miscible with ethanol, ether, benzene, carbon tetrachloride and chloroform. Immiscible with water.
Notes
Light sensitive. Store in a cool place. Incompatible withbases, strong oxidizing agents, copper, peroxides, silver, silver oxides, mercury and mercury oxides.