Search Thermo Fisher Scientific
Thermo Scientific Chemicals
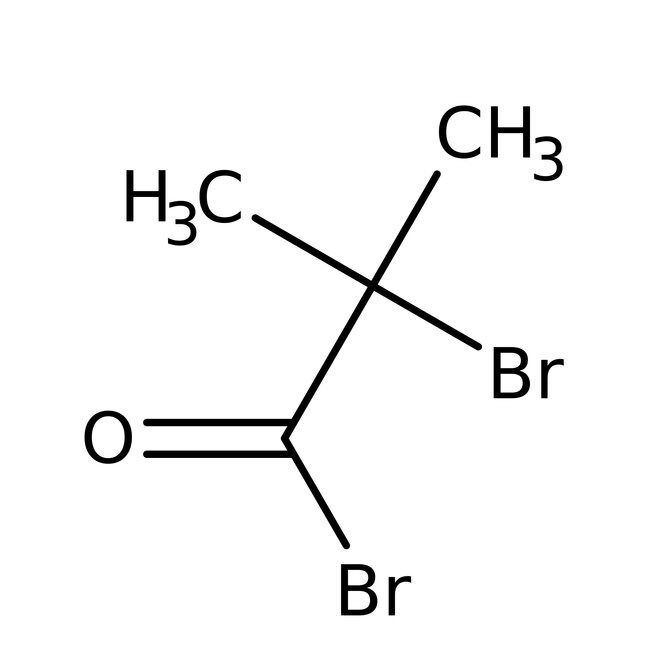
2-Bromoisobutyryl bromide, 97%, Thermo Scientific Chemicals
Catalog number: B24249.22
100 g, Each

Thermo Scientific Chemicals
2-Bromoisobutyryl bromide, 97%, Thermo Scientific Chemicals
Catalog number: B24249.22
100 g, Each
Quantity
Catalog number: B24249.22
also known as B24249-22
Price (USD)
Price: 58.40
Online price: 49.65
Your price:
Quantity
-
Chemical Identifiers
CAS
20769-85-1
IUPAC Name
2-bromo-2-methylpropanoyl bromide
Molecular Formula
C4H6Br2O
InChI Key
YOCIJWAHRAJQFT-UHFFFAOYSA-N
SMILES
CC(C)(Br)C(Br)=O
Specifications
Appearance (Color)
Clear colorless to pale yellow or pale pink
Form
Liquid
Assay (GC)
≥96.0%
Refractive Index
1.5075-1.5125 @ 20?C
Description
2-Bromoisobutyryl bromide is used to prepare N-protected halodienamide, which provides four- and five-membered lactams in the presence of copper(I) and a tertiary amine. Further, it is used as atom transfer radical polymerization(ATRP) initiator for functionalization of hydroxyl groups present on the surface of graphene oxide. It is also used in preparation of polycaprolactone macroinitiators through reaction with oligomeric caprolactone diol and mesoporous silica nanoparticles with ATRP initiator anchored on the exterior surface.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Bromoisobutyryl bromide is used to prepare N-protected halodienamide, which provides four- and five-membered lactams in the presence of copper(I) and a tertiary amine. Further, it is used as atom transfer radical polymerization(ATRP) initiator for functionalization of hydroxyl groups present on the surface of graphene oxide. It is also used in preparation of polycaprolactone macroinitiators through reaction with oligomeric caprolactone diol and mesoporous silica nanoparticles with ATRP initiator anchored on the exterior surface.
Solubility
Miscible with acetone and carbon disulfide.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents and strong alkalis.
2-Bromoisobutyryl bromide is used to prepare N-protected halodienamide, which provides four- and five-membered lactams in the presence of copper(I) and a tertiary amine. Further, it is used as atom transfer radical polymerization(ATRP) initiator for functionalization of hydroxyl groups present on the surface of graphene oxide. It is also used in preparation of polycaprolactone macroinitiators through reaction with oligomeric caprolactone diol and mesoporous silica nanoparticles with ATRP initiator anchored on the exterior surface.
Solubility
Miscible with acetone and carbon disulfide.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents and strong alkalis.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text