Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Sodium peroxide, 95%, Thermo Scientific Chemicals
Catalog number: L13306.18
50 g, Each

Thermo Scientific Chemicals
Sodium peroxide, 95%, Thermo Scientific Chemicals
Catalog number: L13306.18
50 g, Each
Quantity
Catalog number: L13306.18
also known as L13306-18
Price (USD)
Price: 57.50
Your price:
Quantity
-
Chemical Identifiers
CAS
1313-60-6
IUPAC Name
disodium dioxidanediide
Molecular Formula
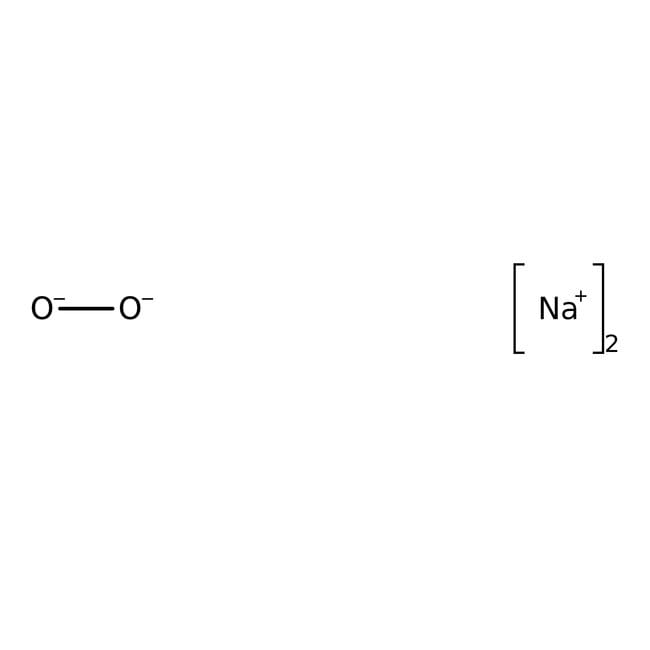
Na2O2
InChI Key
PFUVRDFDKPNGAV-UHFFFAOYSA-N
SMILES
[Na+].[Na+].[O-][O-]
Specifications
Form
Granular solid or beads
Assay (Redox Titration)
≥ 94.0% to ≤ 106.0%
Appearance (Color)
White to yellow
Comment
May contain a small quantity of discoloured material from the manufacturing process.
Description
Sodium peroxide acts as a bleaching agent in laundry and wood pulp. It finds application in the extraction of minerals from various ores. It acts as an oxidizing agent as well as an oxygen source by reacting with carbon dioxide. Furthermore, it is used as a deodorant and an analytical reagent. It is also useful in scuba gear and in submarines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Sodium peroxide acts as a bleaching agent in laundry and wood pulp. It finds application in the extraction of minerals from various ores. It acts as an oxidizing agent as well as an oxygen source by reacting with carbon dioxide. Furthermore, it is used as a deodorant and an analytical reagent. It is also useful in scuba gear and in submarines.
Solubility
Soluble in acid. Insoluble in alkali.
Notes
Air sensitive and hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. It reacts with water violently to give sodium hydroxide and hydrogen peroxide. Incompatible with strong reducing agents, organic materials, alcohols and powdered metals.
Sodium peroxide acts as a bleaching agent in laundry and wood pulp. It finds application in the extraction of minerals from various ores. It acts as an oxidizing agent as well as an oxygen source by reacting with carbon dioxide. Furthermore, it is used as a deodorant and an analytical reagent. It is also useful in scuba gear and in submarines.
Solubility
Soluble in acid. Insoluble in alkali.
Notes
Air sensitive and hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. It reacts with water violently to give sodium hydroxide and hydrogen peroxide. Incompatible with strong reducing agents, organic materials, alcohols and powdered metals.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text