Search Thermo Fisher Scientific
Thermo Scientific Chemicals
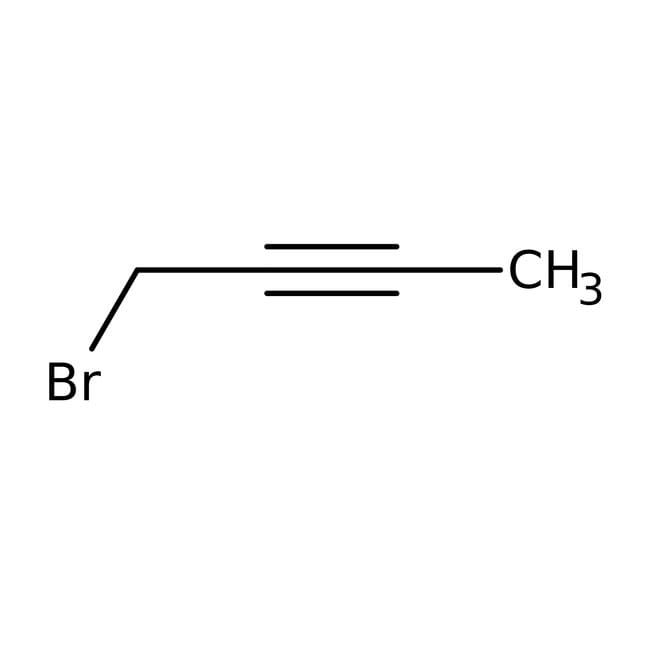
1-Bromo-2-butyne, 98%, Thermo Scientific Chemicals
Catalog number: L20134.06
5 g, Each

Thermo Scientific Chemicals
1-Bromo-2-butyne, 98%, Thermo Scientific Chemicals
Catalog number: L20134.06
5 g, Each
Quantity
Catalog number: L20134.06
also known as L20134-06
Price (USD)
130.00
Each
Quantity
-
Chemical Identifiers
CAS
3355-28-0
IUPAC Name
1-bromobut-2-yne
Molecular Formula
C4H5Br
InChI Key
LNNXOEHOXSYWLD-UHFFFAOYSA-N
SMILES
CC#CCBr
Specifications
Appearance (Color)
Clear colorless to yellow to orange
Refractive Index
1.5060-1.5100 @ 20?C
Form
Liquid
Identification (FTIR)
Conforms
Assay (GC)
≥97.5%
Description
1-Bromo-2-butyne is used in the preparation of six to eight annulated ring compounds in reaction with indoles and pseudopterane (±)-Kallolide B, which is a marine natural product. Further, it acts as a precursor in the preparation of axially chiral teranyl compounds, alkylation of L-tryptophan methyl ester, 4-butynyloxybenzene sulfonyl chloride and mono-propargylated diene derivative. In addition to this, it is also used in the synthesis of isopropylbut-2-ynylamine, allenylcyclobutanol derivatives, allyl-[4-(but-2-ynyloxy)phenyl]sulfane, allenylindium and axially chiral teranyl compounds.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Bromo-2-butyne is used in the preparation of six to eight annulated ring compounds in reaction with indoles and pseudopterane (+/-)-Kallolide B, which is a marine natural product. Further, it acts as a precursor in the preparation of axially chiral teranyl compounds, alkylation of L-tryptophan methyl ester, 4-butynyloxybenzene sulfonyl chloride and mono-propargylated diene derivative. In addition to this, it is also used in the synthesis of isopropylbut-2-ynylamine, allenylcyclobutanol derivatives, allyl-[4-(but-2-ynyloxy)phenyl]sulfane, allenylindium and axially chiral teranyl compounds.
Solubility
Miscible with acetonitrile.
Notes
Incompatible with strong oxidizing agents.
1-Bromo-2-butyne is used in the preparation of six to eight annulated ring compounds in reaction with indoles and pseudopterane (+/-)-Kallolide B, which is a marine natural product. Further, it acts as a precursor in the preparation of axially chiral teranyl compounds, alkylation of L-tryptophan methyl ester, 4-butynyloxybenzene sulfonyl chloride and mono-propargylated diene derivative. In addition to this, it is also used in the synthesis of isopropylbut-2-ynylamine, allenylcyclobutanol derivatives, allyl-[4-(but-2-ynyloxy)phenyl]sulfane, allenylindium and axially chiral teranyl compounds.
Solubility
Miscible with acetonitrile.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text