Search
Preparing Fixed Cells for Labeling

The basics of fixation and permeabilization
Fixing and permeabilizing cells generally locks them in place and makes it possible for larger molecules such as antibodies to access the interior of the cell for better targeting of the protein or condition you're interested in. But, fixed and permeabilized cells are dead, and you lose the ability to look at dynamic biological processes.
The information below will help you decide whether fixation and permeabilization are the right treatments for your investigation.
|
|
Fixation

Figure 1. Formaldehyde fixation essentially locks cellular structures in place.
Fluorescent stains vary in their ability to keep producing a signal after a cell has been fixed. With some stains, you can label cells while they are alive and then fix them without a loss in signal. The more common approach, however, is to fix, permeabilize, and block your cells and then stain them with fluorescent dyes and/or antibody conjugates.
Formaldehyde is the most commonly used fixative; it works by chemically bonding adjacent macromolecules, such as proteins, together. This process is known as crosslinking. Most available formaldehyde preparations are actually paraformaldehyde (PFA, polymeric formaldehyde) dissolved in water or a buffer. The free methanediols in the resulting solution are reactive with amine groups on proteins and other cellular structures that contain nitrogen. PFA also solubilizes some lipids in cellular membranes. PFA is commonly diluted to 3.7–5% v/v and is applied to cells for 10–15 minutes.
While formaldehyde has broad reactivity with a majority of proteins, peptides, and enzymes and is the most commonly used fixative, other approaches can be used in cases where formaldehyde isn’t working for your target. Glutaraldehyde alone can be used as a stronger crosslinking fixative, or can be used in combination with formaldehyde, but glutaraldehyde-treated samples require further processing before antibodies can be used to label the sample. Cold alcohol fixation is sometimes recommended for membrane-surface antigens.
Permeabilization
The permeabilization step removes more cellular membrane lipids to allow large molecules like antibodies to get inside the cell. Thermo Scientific™ Triton™ X-100 and NP-40 are detergents commonly used at 0.1–0.5% (v/v, in PBS) for permeabilization. A permeabilization time of 10–15 minutes is a good starting point, but if that isn’t working well for your target you might need to try a shorter time or a different detergent. These detergents will also permeabilize the nuclear membrane, so they are suitable for a variety of target locations.
Figure 2. The removal of cellular membrane lipids in the permeabilization step allows large molecules access to the interior of the cell.

If you are attempting to visualize a target that is located in a cellular membrane, you may need to skip the permeabilization step completely or try using milder detergents like saponin, Thermo Scientific™ Tween™ 20, or digitonin for shorter times. Try these at 0.1–0.5% (v/v) for 5–10 minutes if Triton X-100 or NP-40 don’t give you good visualization of your target.
Blocking
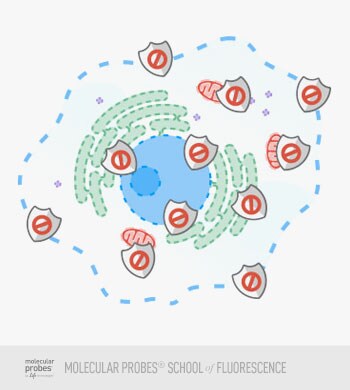
Figure 3. The use of protein-based blocking agents reduces nonspecific staining.
If you are going to use antibodies to label structures in your fixed and permeabilized cells, using a blocking solution before your primary antibody step can be helpful. Blocking is usually performed with a solution containing an excess of protein that serves to reduce the amount of nonspecific binding in your sample. This can be important if your primary or secondary antibody has a tendency to interact with molecules in the sample that are not your target. Reduction of nonspecific “background” staining, most likely due to hydrophobic interactions between the antibody and non-target molecules, will make it easier for you to identify a positive signal and will give you a cleaner end result.
The most common types of blocking solutions for ICC are 3% (w/v) bovine serum albumin in PBS and/or a 10% (v/v) solution of heat-inactivated species-specific serum in PBS, where the serum species matches the species of the secondary antibody. For example, if you are using a mouse anti–rabbit IgG secondary antibody, chose normal heat-inactivated mouse serum to make your blocking reagent.
If you are going to use antibodies to label structures in your fixed and permeabilized cells, using a blocking solution before your primary antibody step can be helpful. Blocking is usually performed with a solution containing an excess of protein that serves to reduce the amount of nonspecific binding in your sample. This can be important if your primary or secondary antibody has a tendency to interact with molecules in the sample that are not your target. Reduction of nonspecific “background” staining, most likely due to hydrophobic interactions between the antibody and non-target molecules, will make it easier for you to identify a positive signal and will give you a cleaner end result. The most common types of blocking solutions for ICC are 3% (w/v) bovine serum albumin in PBS and/or a 10% (v/v) solution of heat-inactivated species-specific serum in PBS, where the serum species matches the species of the secondary antibody. For example, if you are using a mouse anti–rabbit IgG secondary antibody, chose normal heat-inactivated mouse serum to make your blocking reagent. |
If you are going to use antibodies to label structures in your fixed and permeabilized cells, using a blocking solution before your primary antibody step can be helpful. Blocking is usually performed with a solution containing an excess of protein that serves to reduce the amount of nonspecific binding in your sample. This can be important if your primary or secondary antibody has a tendency to interact with molecules in the sample that are not your target. Reduction of nonspecific “background” staining, most likely due to hydrophobic interactions between the antibody and non-target molecules, will make it easier for you to identify a positive signal and will give you a cleaner end result. The most common types of blocking solutions for ICC are 3% (w/v) bovine serum albumin in PBS and/or a 10% (v/v) solution of heat-inactivated species-specific serum in PBS, where the serum species matches the species of the secondary antibody. For example, if you are using a mouse anti–rabbit IgG secondary antibody, chose normal heat-inactivated mouse serum to make your blocking reagent. |

Figure 4. Protein-based blocking agents help reduce non-specific staining. Antibodies are able to displace the blocking proteins to form a high-affinity bond with their epitopes, while blocking proteins prevent low-affinity antibody interactions elsewhere in the sample.
You’ll want to incubate your sample in blocking solution for at least 60 minutes, but it can also be left overnight at room temperature or in the fridge. Once you’re done with the blocking step, if you’re not going to label with antibodies, it’s important to remove the excess blocking solution by washing with PBS.
Troubleshooting
If excess blocking solution is not removed before imaging, it can contribute to nonspecific background fluorescence, which appears as smeary bright signal in all areas of the image.
It can be difficult to figure out where something went wrong in a multi-step process, and immunofluorescence is no exception. The most likely causes of a poor result in immunofluorescence are the primary antibody (either type and/or concentration) or secondary antibody (concentration). See antibody labeling for suggestions on experimental controls to help you optimize your results.
For Research Use Only. Not for use in diagnostic procedures.