Search Thermo Fisher Scientific
RNAi in Non-Mammalian Cultured Cells Large-scale Transcription of Long dsRNA for RNAi
Large mass amounts of ready-to-use dsRNA for RNAi experiments in non-mammalian systems can now be easily prepared using the MEGAscript™ RNAi Kit. The kit is based on Ambion's patented high-yield transcription technology and includes reagents for transcription, nuclease digestion and dsRNA clean up. The kit incorporates a single step, high-yield transcription/annealing reaction, followed by removal of DNA, ssRNA, proteins, free nucleotides and buffer. Each kit can generate 1-2 mg of ready-to-use dsRNA.
Large Scale Synthesis and Purification of dsRNA
Long double-stranded RNA (dsRNA) has been used extensively to induce RNA interference (RNAi) in C. elegans and Drosophila melanogaster (see sidebar, "siRNA or dsRNA"). The ability to generate large amounts of clean dsRNA is essential for the success of RNAi experiments in these organisms. The MEGAscript RNAi Kit, based on Ambion's patented MEGAscript large scale transcription technology, allows RNAi researchers to synthesize 50-100 µg amounts of dsRNAs of various sizes in a single reaction. PCR or plasmid DNA templates containing a T7 RNA polymerase promoter can be used to generate RNA transcripts. DNA templates containing T7 RNA polymerase promoters on both ends of the inserted sequence can also be used (Figure 1). These latter templates eliminate the need for a separate annealing step the simultaneous transcription from both promoters results in the annealing of ssRNAs in the reaction tube.
To ensure that any effects seen in an RNAi experiment are due to the dsRNA itself, it is imperative that the RNA be purified from any contaminants carried over from the transcription reaction. Unlike less complete competing products, the MEGAscript RNAi Kit provides all the reagents necessary to eliminate DNA template, single-stranded RNA (ssRNA), proteins and free nucleotides. After the dsRNA is synthesized, it is digested with DNase I and ssRNA specific RNase (included) to degrade the DNA template and any residual ssRNA, respectively. The dsRNA is then purified away from proteins, free nucleotides, and nucleic acid degradation products using a quick glass fiber filter-based procedure. The kit also comes with a comprehensive Instruction Manual and a control template to assess transcriptional efficiency. Up to 2 mg of dsRNA can be produced from a single kit.

Figure 1. MEGAscript RNAi Kit Procedure.
To ensure that any effects seen in an RNAi experiment are due to the dsRNA itself, it is imperative that the RNA be purified from any contaminants carried over from the transcription reaction. Unlike less complete competing products, the MEGAscript RNAi Kit provides all the reagents necessary to eliminate DNA template, single-stranded RNA (ssRNA), proteins and free nucleotides. After the dsRNA is synthesized, it is digested with DNase I and ssRNA specific RNase (included) to degrade the DNA template and any residual ssRNA, respectively. The dsRNA is then purified away from proteins, free nucleotides, and nucleic acid degradation products using a quick glass fiber filter-based procedure. The kit also comes with a comprehensive Instruction Manual and a control template to assess transcriptional efficiency. Up to 2 mg of dsRNA can be produced from a single kit.
Analysis of the RNAi Effect
Ambion has developed a complete line of products and a unique web resource for the design, execution and analysis of RNAi experiments. Our kits and reagents have been developed and are supported by scientists at Ambion who are actively performing silencing experiments of their own (Jarvis and Ford, 2001; Brown et al., 2002; Byron et al., 2002). Here we have tested and optimized various parameters of the RNAi effect in cultured Drosophila melanogaster cells. The MEGAscript RNAi Kit was used to generate full-length dsRNA to the Drosophila hrp48 and U2AF50 genes from the corresponding cDNA constructs. As no transfection is required for insect cells, 1x106 Schneider's Drosophila L2 cells were grown in 6 well plates in serum free medium and directly treated with 10 nM of dsRNA. Western blot analysis 72 hours after treatment showed that hrp48 and U2AF50 protein expression levels were reduced by 98% and 80%, respectively (Figure 2A). The silencing effect was specific and dose-dependent (Figure 2A and 2C). A strong correlation between suppression of mRNA and protein expression was demonstrated by Northern blot analysis (Figure 2A and 2B). This observation supports the model that dsRNA-triggered gene silencing results from a reduction in the amount of target mRNA available for translation.
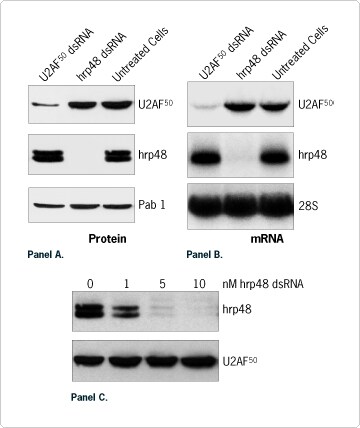
Figure 2. dsRNA-Mediated Silencing of Drosophila U2AF50 and hrp48 Gene Expression. (A) Specific reduction of protein expression levels. The RNAi effect was analyzed by Western blot with anti-U2AF50 (50 kDa) and anti-hrp48 (48 kDa; migrates as a doublet) antibodies. An antibody directed against Pab 1 (lower panel) was used as an additional negative control. (B) Specific reduction of mRNA expression levels. Total RNA samples were purified and analyzed using by Northern blotting. Radiolabeled RNA probes were produced from two 300 bp long PCR templates (U2AF50 and hrp48) or from Ambion pTRI RNA 28S plasmid. The latter probe was used to normalize the amount of RNA added. (C) Dose sensitive reduction of hrp48 protein expression.

Figure 2. dsRNA-Mediated Silencing of Drosophila U2AF50 and hrp48 Gene Expression. (A) Specific reduction of protein expression levels. The RNAi effect was analyzed by Western blot with anti-U2AF50 (50 kDa) and anti-hrp48 (48 kDa; migrates as a doublet) antibodies. An antibody directed against Pab 1 (lower panel) was used as an additional negative control. (B) Specific reduction of mRNA expression levels. Total RNA samples were purified and analyzed using by Northern blotting. Radiolabeled RNA probes were produced from two 300 bp long PCR templates (U2AF50 and hrp48) or from Ambion pTRI RNA 28S plasmid. The latter probe was used to normalize the amount of RNA added. (C) Dose sensitive reduction of hrp48 protein expression.
Visualizing Introduced dsRNA in Cells
One way to further understand the mechanism of RNAi is to track the introduced dsRNA in live cells. Fluorescent labeling is commonly used by scientists to localize macromolecules, but it was not known whether the incorporation of a fluorophore into dsRNA would affect its ability to induce gene silencing. To address this question, we used Ambion's Silencer™ siRNA Labeling Kit to couple the fluorescent label, Cy™3, to long dsRNA prepared with the MEGAscript RNAi Kit and then compared labeled dsRNA activity to that of unlabeled. Figure 3A shows that the same powerful silencing effect was obtained with both labeled and unlabeled dsRNA specific for hrp48 or U2AF50 gene products. No cytotoxic effect was associated with the fluorescent moiety and the expression level of a non-related control protein, Pab 1, was not affected (Figure 3A). The specific silencing of hrp48 and U2AF50 proteins was also confirmed by immunofluorescence microscopy (data not shown). Direct observation of the Cy3 fluorescence demonstrated that both hrp48 and U2AF50 labeled dsRNA were taken up equivalently into L2 cells and localized to discrete perinuclear foci in the cytoplasm (Figure 3B). This localization is very similar to what was previously reported for siRNA in mammalian cells (Byron et al., 2002). Finally time course studies showed that the RNAi effect was maintained in cultured Drosophila cells for up to 10 days and that labeled dsRNA was passed on to daughter cells during cell division (data not shown).
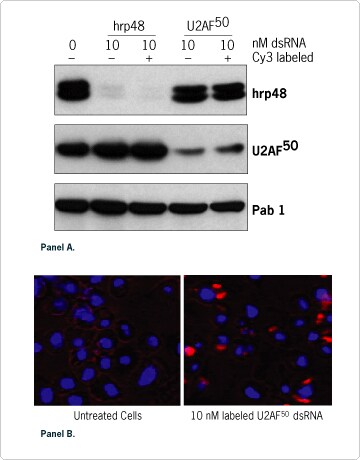
Figure 3. Fluorescently Labeled dsRNA Retains Functionality. (A) Silencing experiments and Western blot analyses were performed as described in Figure 2 three days after treatment with 10 nM of the indicated labeled or unlabeled dsRNA. (B) dsRNA specific for U2AF50 was labeled with Cy3 using the Silencer™ siRNA Labeling Kit, introduced into Drosophila L2 cells, and the cells were analyzed by fluorescence microscopy. BLUE: DAPI stained nuclei. RED: Cy3 labeled U2AF50 dsRNA.
The Silencer Cy3 and FAM siRNA Labeling Kits can be used to label siRNA and long dsRNA, and are the perfect complement to the MEGAscript™ RNAi Kit. Labeled dsRNA can be used to analyze subcellular localization, stability, and uptake. In addition, fluorescently labeled dsRNA is particularly well-suited for double label experiments (with a labeled antibody) to track cells that take up dsRNA and to correlate uptake with down-regulation of the target protein. Although the Silencer siRNA Labeling Kits were developed for labeling siRNA, they can be readily adapted for labeling long dsRNA.
References
1. Brown D, Jarvis R, Pallotta V, Byrom M, and Ford L. (2002) RNA Interference in Mammalian Cell Culture: Design, Execution and Analysis of the siRNA Effect. Ambion TechNotes 9(1):3 5.
2. Jarvis R and Ford L (2001) The siRNA Target Site is an Important Parameter for Inducing RNAi in Human Cells. Ambion TechNotes 8(5):3 5.
3. Byron M, Pallotta V, Brown B, and Ford L. (2002) Visualizing siRNA in Mammalian Cells: Fluorescence Analysis of the RNAi Effect. Ambion TechNotes 9(3):6 8.
Cy™3 is a trademark of Amersham Biosciences.
The Cy3 and Fluorescein Labeling reagents manufactured for Ambion by Mirus Corporation.

Figure 3. Fluorescently Labeled dsRNA Retains Functionality. (A) Silencing experiments and Western blot analyses were performed as described in Figure 2 three days after treatment with 10 nM of the indicated labeled or unlabeled dsRNA. (B) dsRNA specific for U2AF50 was labeled with Cy3 using the Silencer™ siRNA Labeling Kit, introduced into Drosophila L2 cells, and the cells were analyzed by fluorescence microscopy. BLUE: DAPI stained nuclei. RED: Cy3 labeled U2AF50 dsRNA.
The Silencer Cy3 and FAM siRNA Labeling Kits can be used to label siRNA and long dsRNA, and are the perfect complement to the MEGAscript™ RNAi Kit. Labeled dsRNA can be used to analyze subcellular localization, stability, and uptake. In addition, fluorescently labeled dsRNA is particularly well-suited for double label experiments (with a labeled antibody) to track cells that take up dsRNA and to correlate uptake with down-regulation of the target protein. Although the Silencer siRNA Labeling Kits were developed for labeling siRNA, they can be readily adapted for labeling long dsRNA.
References
1. Brown D, Jarvis R, Pallotta V, Byrom M, and Ford L. (2002) RNA Interference in Mammalian Cell Culture: Design, Execution and Analysis of the siRNA Effect. Ambion TechNotes 9(1):3 5.
2. Jarvis R and Ford L (2001) The siRNA Target Site is an Important Parameter for Inducing RNAi in Human Cells. Ambion TechNotes 8(5):3 5.
3. Byron M, Pallotta V, Brown B, and Ford L. (2002) Visualizing siRNA in Mammalian Cells: Fluorescence Analysis of the RNAi Effect. Ambion TechNotes 9(3):6 8.
Cy™3 is a trademark of Amersham Biosciences.
The Cy3 and Fluorescein Labeling reagents manufactured for Ambion by Mirus Corporation.
siRNA or dsRNAPost-transcriptional gene silencing (PTGS) or RNA interference (RNAi) is the phenomenon in which introduction of a double-stranded RNA (dsRNA) suppresses the expression of the homologous gene. dsRNA molecules are reduced in vivo to 21-23 nt small interfering RNAs (siRNAs) which are the mediators of the RNAi effect. RNAi has been successfully used to reduce gene expression in a variety of organisms including zebrafish, nematodes (C. elegans), insects (Drosophila melanogaster), planaria, cnidaria, trypanosomes, mice and mammalian cells. In C. elegans and Drosophila, RNAi is typically induced by the introduction of long dsRNA (up to 1-2 kb) produced by in vitro transcription. This simple approach cannot be used in mammalian cells, where introduction of long dsRNA elicits a strong antiviral response obscuring any gene-specific silencing effect. However, introduction of 21 nt siRNAs with 2 nt 3' overhangs does not stimulate the anti-viral response in mammalian cells and can effectively target specific mRNAs for gene silencing. Such molecules can be prepared by chemical synthesis or in vitro transcription (e.g. using Ambion's
siRNA Oligonucleotide Synthesis Service or the
Silencer™ siRNA Construction Kit, respectively). Alternatively, they can be expressed in vivo using a specially designed siRNA expression vector.